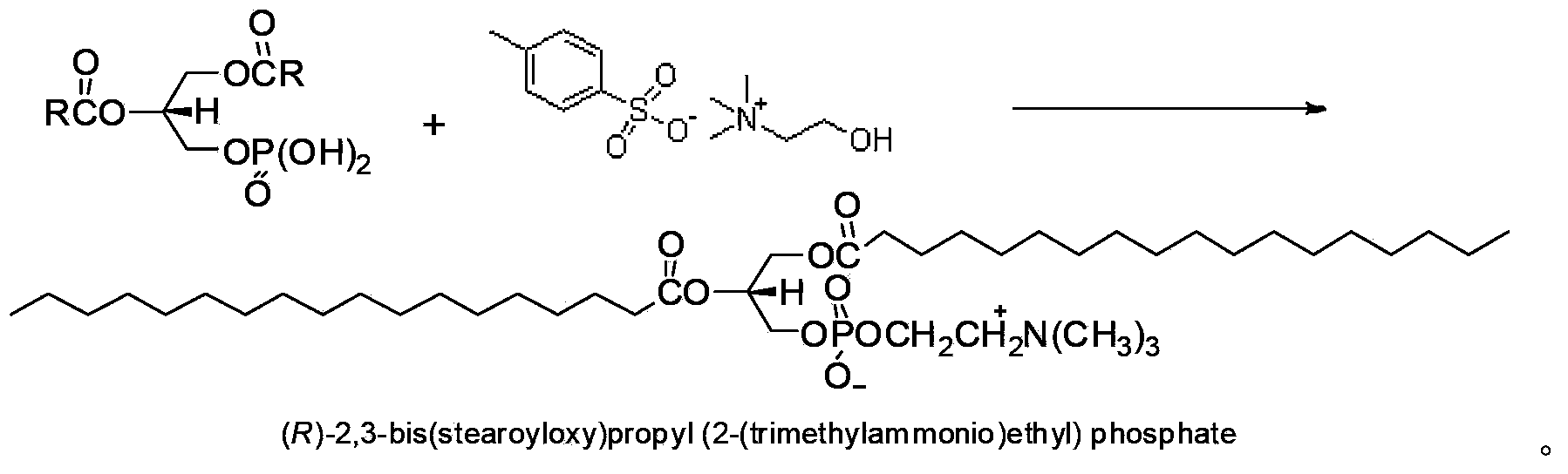
Preparation method of (R)-1,2-distearoyl phosphatidylcholine
A technology of stearoyl phosphatidyl choline and distearoyl, which is applied in the field of preparation of (R)-1,2-distearoyl phosphatidyl choline, which can solve the problem of unsuitability for industrial production and cumbersome reaction steps, etc. problems, to achieve the effects of industrial application, simplification of reaction steps, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1, the preparation of (S)-1,2-diol-3-iodopropane
[0038] Add 0.08 g (0.1 mmol) of hydroquinidine 1,4-(2,3-naphthyridine) diether (DHQD) into a round bottom reaction flask 2 PHAL, 0.018g (0.05mmol) K 2 OSo 2 (OH) 4 , 9.8 g (30 mmol) K 3 Fe(CN) 6 , 4.2 g (30 mmol) K 2 CO 3 , 2.5 g (30 mmol) NaHCO 3 , 0.95g (10mmol) and 100mL of an aqueous solution of tert-butanol (tert-butanol: water = 1:1), stirred well at 0°C, then added 1.7g (10mmol) of 3-iodopropene to react for 6 hours, and added 10g Na 2 S 2 o 4And continue stirring for 30 minutes to stop the reaction. The aqueous layer was separated and extracted with ethyl acetate, and the combined organic layers were washed with 1N KOH, dilute hydrochloric acid and saturated brine, respectively. Dried over anhydrous magnesium sulfate and concentrated to obtain a crude product. Recrystallized from a mixed solvent of petroleum ether and methanol to obtain 1.58 g of (S)-1,2-diol-3-iodopropane with a yield of 7...
Embodiment 2
[0039] Embodiment 2, the preparation of (R)-1,2-distearoyl-3-iodopropane
[0040] Stearic anhydride (0.8g, 1.5mmol), (S)-1,2-diol-3-iodopropane (0.2g, 1mmol) was added to 50mL of dichloromethane, stirred and refluxed at 40°C under nitrogen protection for 3 Hours, stop heating, after cooling to room temperature, pour 50mL of anhydrous diethyl ether. The organic layer was washed with 10 mL of 5% aqueous sodium bicarbonate solution and 10 mL of saturated brine, dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure to obtain a crude product that was recrystallized with ethanol to obtain 0.61 g of (S)-1,2-diol-3 - iodopropane, yield 83%.
Embodiment 3
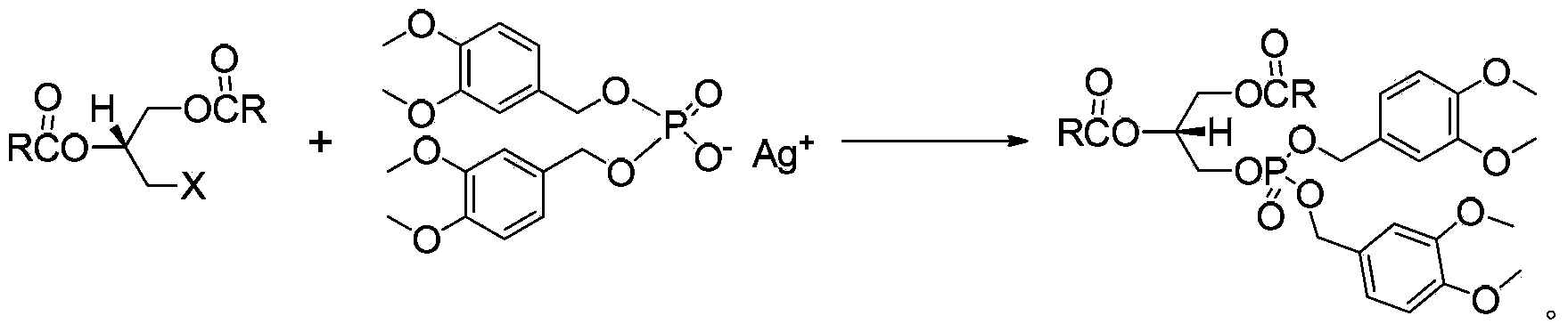
[0041] Example 3, Preparation of (R)-1,2-distearoyl-3-(3,4-dimethoxybenzyloxy)phosphate propane
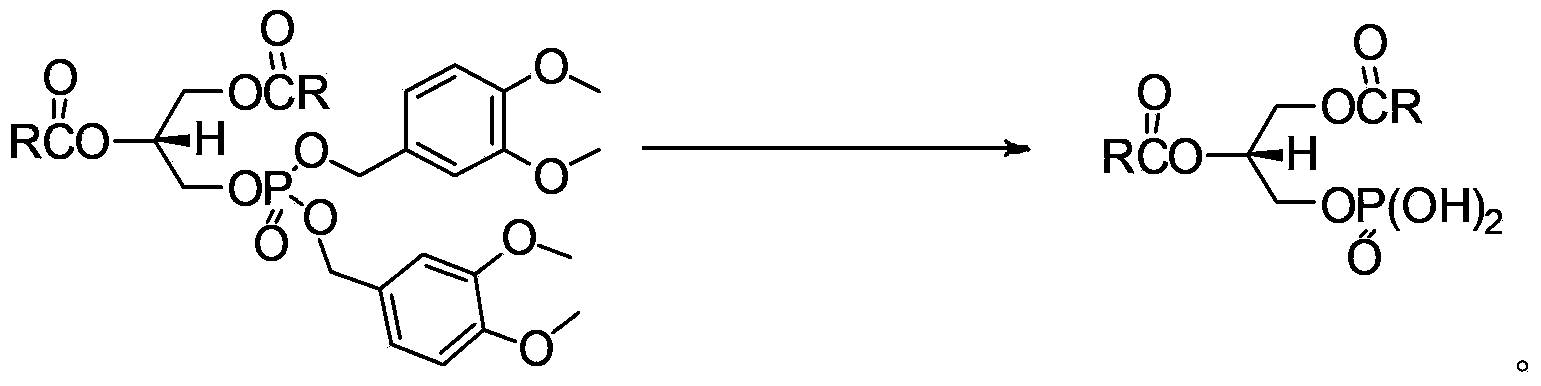
[0042] (R)-1,2-Distearoyl-3-iodopropane (0.6g, 0.8mmol) and 3,4-dimethoxybenzyl silver phosphate (1.3g, 2.5mmol) were added under dark conditions into 50 mL of toluene, reflux for 6 h to stop the reaction, cool to room temperature, filter, wash with dichloromethane, combine the organic layers and concentrate under reduced pressure, and recrystallize the obtained crude product with ethanol to obtain 0.52 g of a white solid, with a yield of 65%.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap