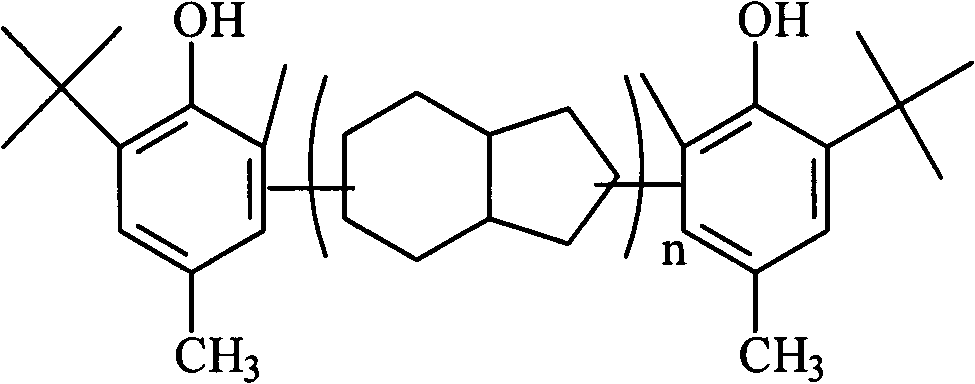
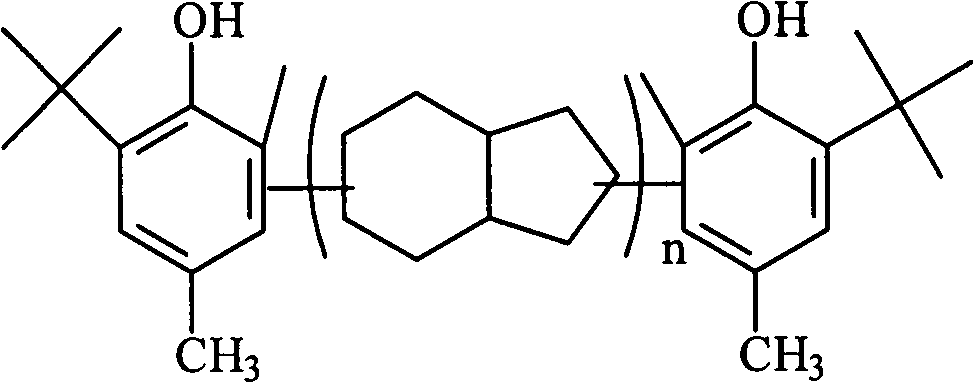
Preparation method for polymerization-type asymmetric hindered phenol anti-oxidant resins
A technology of asymmetric hindered phenol and antioxidant, which is applied in the field of preparation of polymerized asymmetric hindered phenolic antioxidant resin, and can solve the problems of synthesis process pollution, antioxidant activity and thermal stability of antioxidants, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] In the 500ml reactor equipped with stirrer, reflux condenser, feeding device and nitrogen protection device, add 100g toluene, 100g p-cresol, 2g boron trifluoride complex catalyst, after replacing with nitrogen, heat to 85 ℃, add 80g of dicyclopentadiene dropwise with a pump, and the dropping time is 60min. After the dropwise addition, the temperature is raised to 132°C. After reacting for 3h, the reaction solution is washed with alkali and washed with water, and the unreacted p-methyl group is distilled off under reduced pressure. Phenol, then add 100g of solvent toluene, 1.5g of phosphoric acid, 10g of alkylation catalyst, reaction temperature 76°C, dropwise add 20g of methanol and isobutanol molar ratio 3:7 mixed raw materials, dropwise time 20min, after dropwise addition, react After 2 hours, after alkali washing and water washing, the solvent and unreacted raw materials were distilled off under reduced pressure, distilled to 216° C., and 146 g of the product was obt...
Embodiment 2
[0020] In the 500ml reactor equipped with stirrer, reflux condenser, feeding device and nitrogen protection device, add 100g toluene, 100g p-cresol, 3g boron trifluoride complex catalyst, after replacing with nitrogen, heat to 90 ℃, use a pump to add 120g of dicyclopentadiene dropwise for 30 minutes, after the dropwise addition, raise the temperature to 140°C, and react for 3 hours, the reaction solution is washed with alkali, and after washing with water, the unreacted p-methyl group is distilled off under reduced pressure. Phenol, add 100g of solvent toluene, 1.5g of phosphoric acid, 12g of alkylation catalyst, reaction temperature 80 ℃, dropwise add methanol and isobutanol molar ratio 5: 5 mixed raw materials, dropwise time 22min, react after dropwise After 2 hours, after alkali washing and water washing, the solvent and unreacted raw materials were distilled off under reduced pressure, distilled to 262° C., and 182 g of the product was obtained after cooling. The calculated...
Embodiment 3
[0022] In the 500ml reactor equipped with stirrer, reflux condenser, feeding device and nitrogen protection device, add 100g toluene, 100g p-cresol, 2g boron trifluoride complex catalyst, after replacing with nitrogen, heat to 83 ℃, use a pump to add 100g of dicyclopentadiene dropwise, and the dropping time is 60min. After the dropwise addition, the temperature is raised to 125°C. After 3 hours of reaction, the reaction solution is washed with alkali, and after washing with water, the unreacted p-methyl group is distilled off under reduced pressure. Phenol, then add 100g of solvent toluene, 1.5g of phosphoric acid, 10g of alkylation catalyst, reaction temperature 80°C, dropwise add 20g of ethanol and isobutanol molar ratio 3:7 mixed raw materials, dropwise time 20min, after dropwise addition, react After 2.5 hours, after alkali washing and water washing, the solvent and unreacted raw materials were distilled off under reduced pressure, distilled to 231° C., and 172 g of the pro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| softening point | aaaaa | aaaaa |
| softening point | aaaaa | aaaaa |
| softening point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com