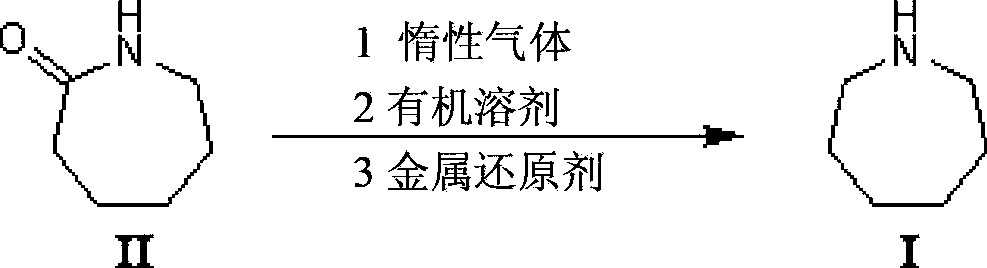
Method for synthesizing azacycloheptane
An azacycloheptane and a synthesis method technology, applied in the direction of organic chemistry and the like, can solve the problems of decreased product yield and purity, difficult commercial reduction system, poor reduction effect, etc., to reduce purification costs and facilitate large-scale industrialization Application, the effect that is beneficial to environmental protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Put 150mL of anhydrous tetrahydrofuran in a dry reaction bottle, cool down to 0°C, and add 33.3g of anhydrous aluminum trichloride (0.25mol) and 10.0g of sodium borohydride (0.26mol) in turn after argon replacement, and wait for the reaction liquid to naturally After raising the temperature to 25°C, stir for 1 hour, add 11.3g of caprolactam (0.1mol) and raise the temperature of the reaction solution to 50°C, reflux for 8 hours, then cool the reaction solution to 0°C, add 500mL of water, stir until the reaction solution is clear, and use 2M Potassium hydroxide solution adjusts the pH to ≥ 12, extracts the reaction solution 3 times with 300 mL of dichloromethane and combines the organic phases, washes the organic phase with 60 mL of saturated brine, dries over anhydrous magnesium sulfate, concentrates the filtrate to recover the solvent to obtain a crude product, usually The crude product was distilled under pressure, and the fractions between 137 and 140°C were collected ...
Embodiment 2
[0033] Put 150mL of anhydrous tetrahydrofuran in a dry reaction bottle, cool down to 0°C, add 36.1g of anhydrous aluminum trichloride (0.27mol) and 15.2g of sodium borohydride (0.28mol) in turn after nitrogen replacement, and wait for the reaction liquid to heat up naturally After reaching 25°C, stir for 1 hour, add 11.3g caprolactam (0.1mol) and raise the temperature of the reaction solution to 55°C, reflux for 8 hours, then cool the reaction solution to 0°C, add 500mL of water, stir until the reaction solution is clear, and oxidize with 2M hydroxide Adjust the pH of the sodium solution to ≥12, extract the reaction solution 3 times with 300 mL of dichloromethane and combine the organic phases, wash the organic phase with 60 mL of saturated brine, dry over anhydrous magnesium sulfate, concentrate the filtrate to recover the solvent to obtain a crude product, and reduce The crude product was distilled under pressure, and the fraction between 54 and 58°C was collected to obtain 9...
Embodiment 3
[0035] Put 150mL of anhydrous tetrahydrofuran in a dry reaction bottle, cool down to 0°C, add 36.1g of anhydrous aluminum trichloride (0.27mol) and 15.2g of sodium borohydride (0.28mol) in turn after nitrogen replacement, and wait for the reaction liquid to heat up naturally After reaching 25°C, stir for 1 hour, add 11.3g of caprolactam (0.1mol), introduce nitrogen to raise the pressure to 1520mmHg and raise the temperature of the reaction solution to 65°C, reflux for 10h, then cool the reaction solution to 0°C, add 500mL of water, and stir until the reaction The liquid was clarified, and the pH was adjusted to ≥12 with 2M sodium hydroxide solution, the reaction liquid was extracted 3 times with 300 mL of dichloromethane and the organic phase was combined, washed with 60 mL of saturated brine, dried over anhydrous sodium sulfate, and the filtrate was concentrated and recovered The solvent was used to obtain a crude product, which was distilled under reduced pressure at 40 mmHg,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com