The invention discloses cotton polyamine oxidase GhPAO2 gene and application thereof
A polyamine oxidase, gene technology, applied in the field of genetic engineering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 Cloning and Sequence Analysis of Cotton Polyamine Oxidase GhPAO2 Coding cDNA
[0025] 1.1 Cloning of cotton polyamine oxidase GhPAO2 cDNA
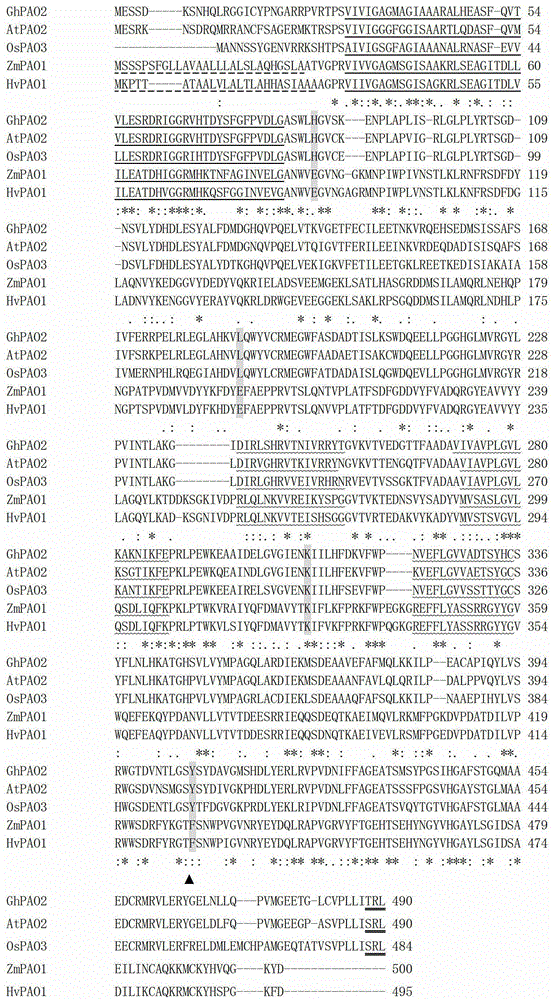
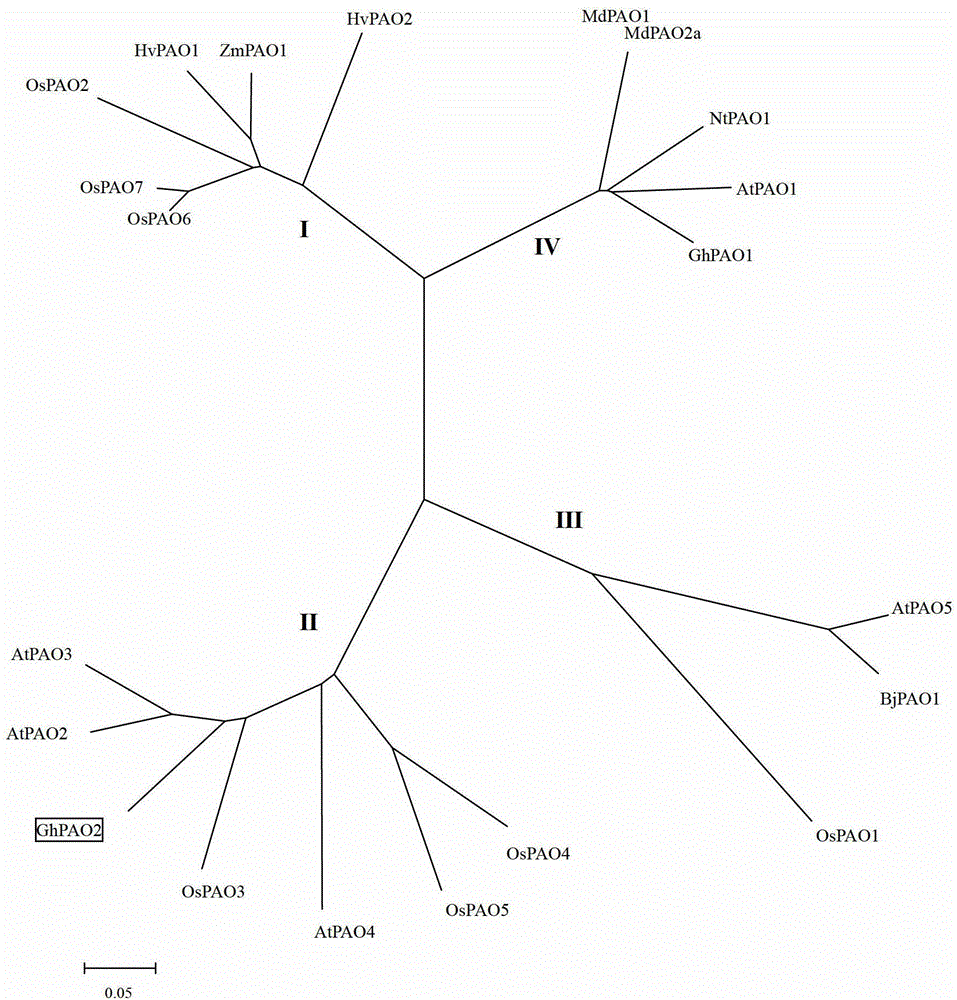
[0026] Using the cDNA that was not inoculated with Verticillium dahliae as the driver, and the induced 'Jimian 20' cDNA as the tester, follow the subtraction kit BD PCR Select from Clontech Company TM The instructions for the cDNA Subtraction Kit perform suppression subtraction hybridization. The cDNA obtained by SSH was connected with pGEM-T Easy Vector (Beijing Tianwei Times Technology Co., Ltd.), and then transferred into E. coli competent cells Top10 by heat shock method. The positive clones were selected and sequenced to construct upland cotton varieties resistant to Verticillium wilt SSH library. Based on an EST sequence in the library, a cDNA sequence was obtained through homologous comparison and bioinformatics analysis of the EST database on the NCBI website, which was presumed to be the cDNA sequence encoding ...
Embodiment 2
[0048] Example 2 Expression Analysis of Cotton Polyamine Oxidase GhPAO2 in Escherichia coli
[0049] 1.1 Construction of prokaryotic expression recombinant vector
[0050] The pCold TF plasmid and the pMD18-T plasmid (pMD18-GhPAO2) containing the mORF of the GhPAO2 gene were subjected to a double enzyme digestion reaction with KpnI and XbaI respectively. The enzyme digestion reaction system is as follows:
[0051]
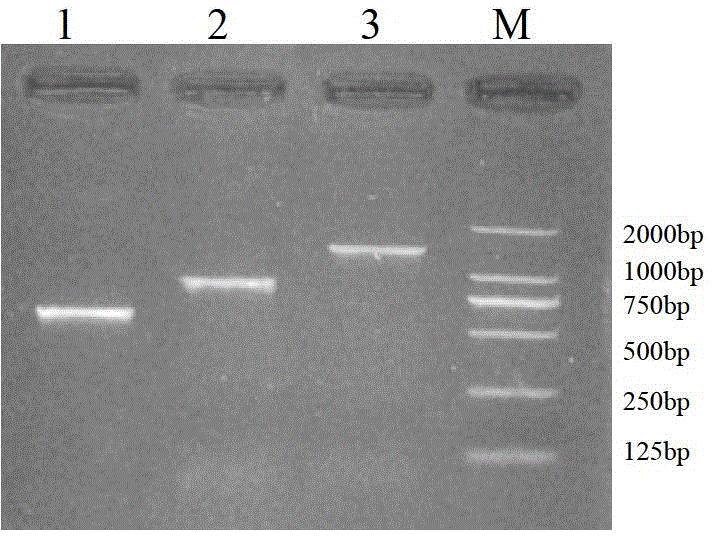
[0052] Carry out agarose gel electrophoresis on the digested products, and recover the corresponding digested fragments respectively according to the instructions of the EZ Spin Column DNA Gel Extraction Kit UN1Q-10 column DNA gel recovery kit from Shanghai Bioengineering Co., Ltd., and then incubate at 16°C After ligation by T4 DNA ligase for 12 hours, 10 μL ligation reaction system is as follows:
[0053]
[0054] The ligation product was transformed into competent cells DH5α. After heat shock transformation, the positive clones were screened and sent to S...
Embodiment 3
[0062] Example 3 Analysis of Cotton Polyamine Oxidase GhPAO2 Catalytic Properties in Vitro
[0063] 1.1GhPAO2 is a flavoprotein with FAD as prosthetic group
[0064] Existing studies have proved that PAO is a type of flavoprotein with FAD as a prosthetic group. Through non-covalent bonding, FAD acts as an electron donor to oxidize oxygen molecules in polyamine oxidase and stimulate the oxidation activity of PAO. Sequence comparison analysis shows that the N-terminus of the GhPAO2 protein has a FAD binding fingerprint pattern. In order to verify whether the purified protein uses FAD as a prosthetic group, the present invention takes the purified protein solution, adds 5% chloroform and shakes vigorously, and then centrifuges at 12,000 rpm for 10 minutes at 4°C. , take the supernatant and continuously measure the absorption value at 300-540nm on the Shimadzu UV-1750 UV-Vis spectrophotometer, which shows that two typical FAD protein absorption peaks appear at 380 and 460nm ( Fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com