Modifying method of ternary anode material
A positive electrode material and modification technology, which is applied in the direction of electrical components, battery electrodes, circuits, etc., can solve the problems of cycle performance deterioration, unfavorable slurry dispersion and stirring, and collapse of surface layered structure, so as to reduce battery bulging and good cycle performance, pH lowering effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
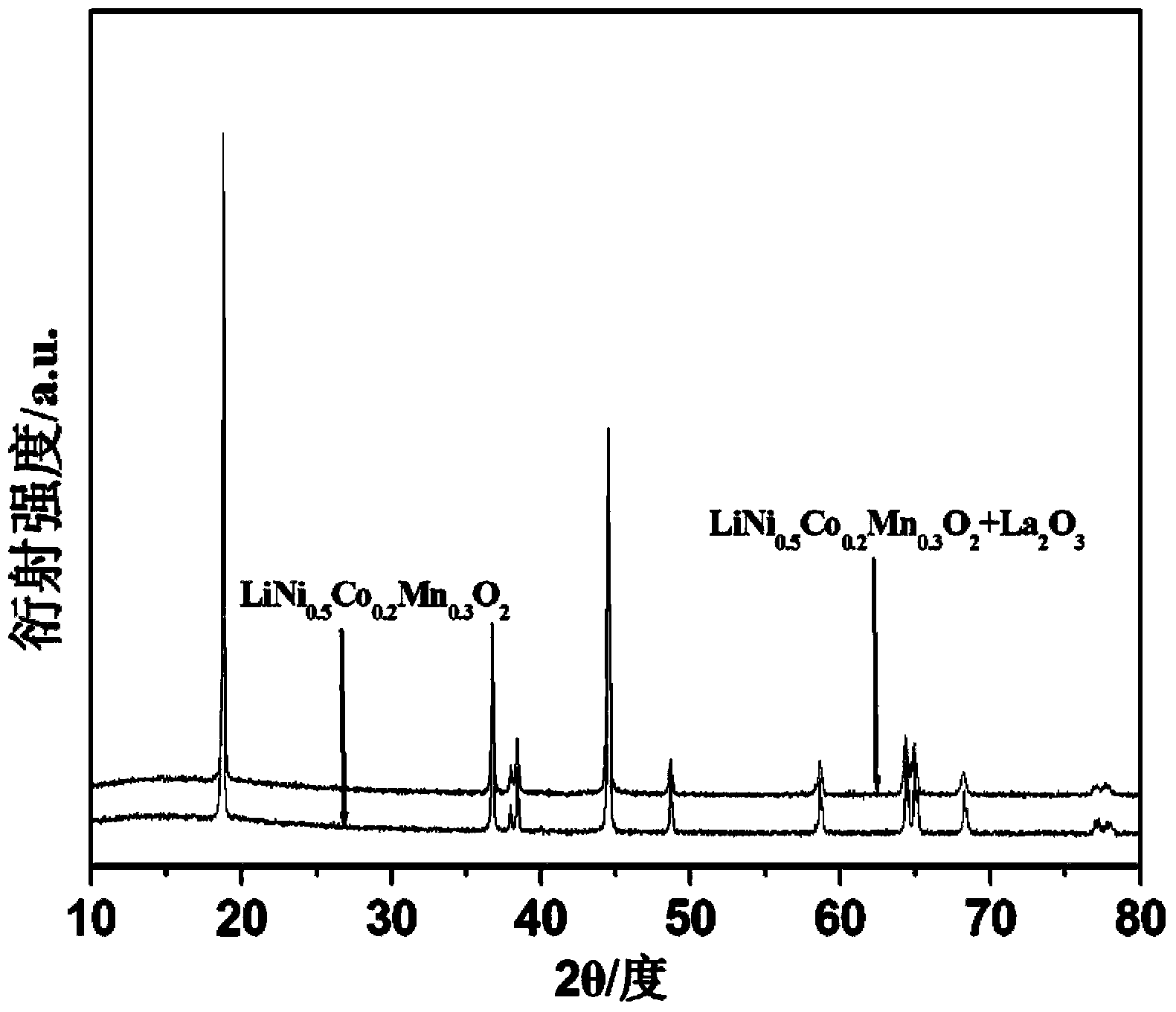
[0025] Will LiNi 1 / 3 co 1 / 3 mn 1 / 3 o 2 Weigh 100g and add it into 100ml of 0.05 mol / L acetic acid solution, stir magnetically for 30min, dry after washing. Add 0.59g of lanthanum oxide to the dried material, the mass of the lanthanum element is 0.5% of the mass of the ternary positive electrode material, then add an appropriate amount of distilled water, stir evenly, form a slurry, and put it into a sagger. Sinter directly at 600° C. for 4 hours in an air atmosphere, cool to room temperature, and grind through a 400-mesh sieve to obtain a lanthanum oxide-coated ternary cathode material.
[0026] The lanthanum oxide-coated LiNi prepared in this example 1 / 3 co 1 / 3 mn 1 / 3 o 2 , using a metal lithium sheet as the counter electrode, and assembled into a CR2032 button battery in a glove box. The charge and discharge voltage range is 3.0~4.4V. Under the charge and discharge rate of 0.1C, the first charge and discharge capacity of the fabricated battery reaches 193 mAh / g and 1...
Embodiment 2
[0028] Will LiNi 0.5 co 0.2 mn 0.3 o 2 Weigh 100g and add to 100ml of 0.08 mol / L acetic acid solution, stir magnetically for 30min, and dry after washing. Add 2.17g of lanthanum carbonate octahydrate to the dried material, the mass of the lanthanum element is 1.0% of the mass of the ternary positive electrode material, then add an appropriate amount of distilled water, stir evenly, form a slurry, and put it into a sagger. Sinter directly at 900° C. for 6 hours in an air atmosphere, cool to room temperature, and grind through a 400-mesh sieve to obtain a lanthanum oxide-coated ternary positive electrode material.
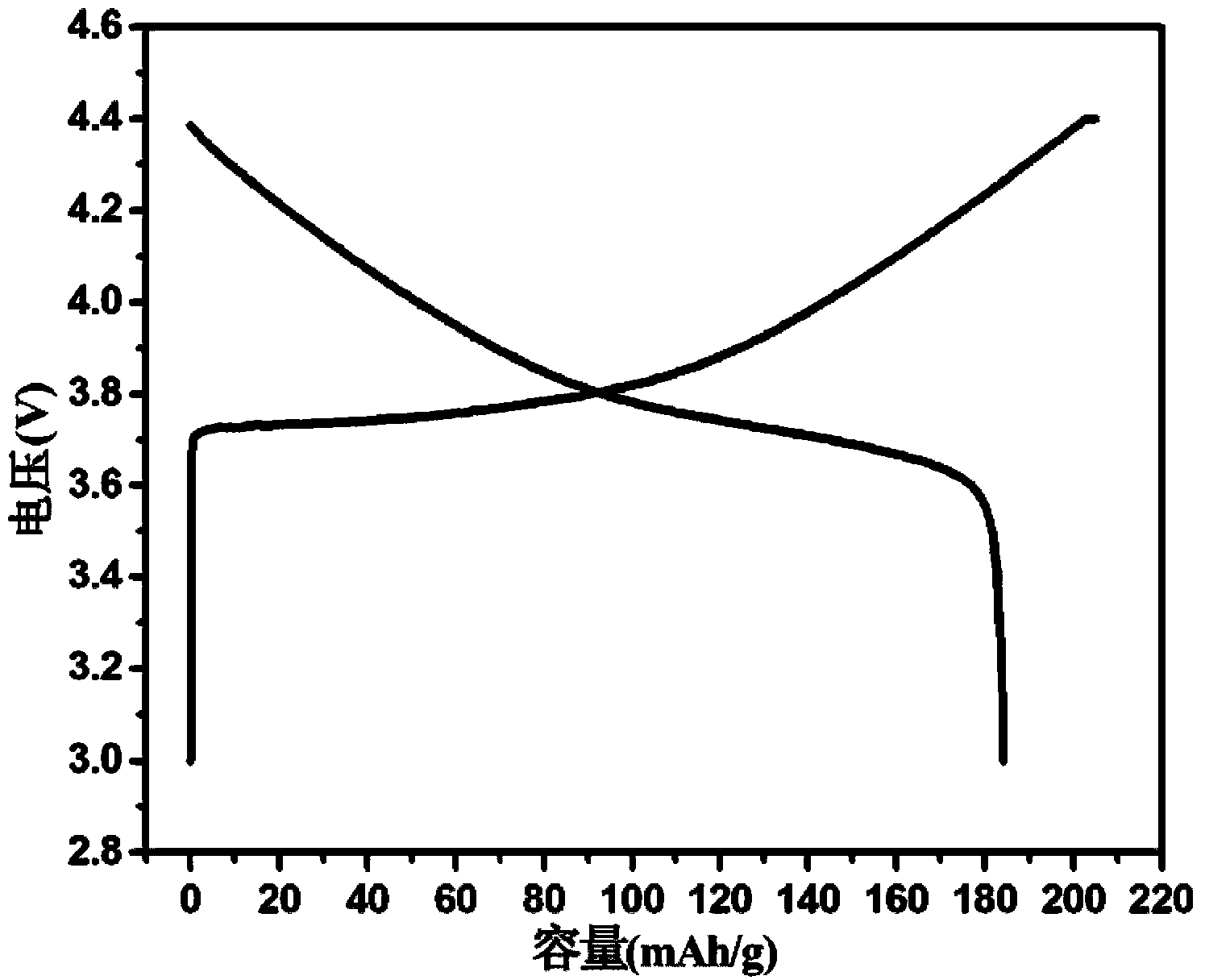
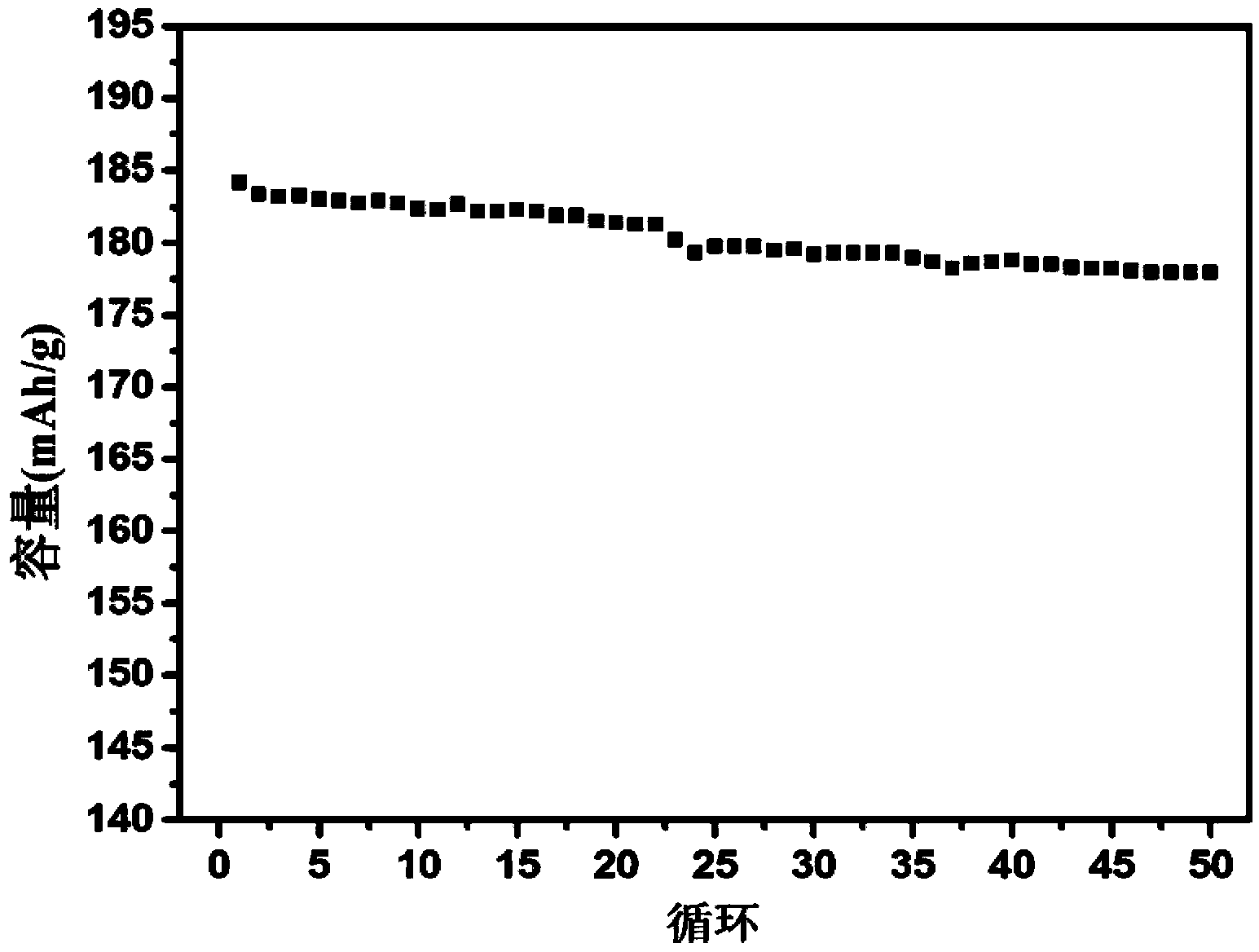
[0029] The lanthanum oxide-coated LiNi prepared in this example 0.5 co 0.2 mn 0.3 o 2 The positive electrode material was assembled into a battery. The specific method was the same as in Example 1. Under the charge and discharge rate of 0.1C, the initial charge and discharge capacity reached 205 mAh / g and 184 mAh / g, and the initial charge and discharge efficie...
Embodiment 3
[0031] Will LiNi 0.5 co 0.2 mn 0.3 o 2 Weigh 100g and add it to 100ml of 0.11 mol / L acetic acid solution, stir magnetically for 30min, and dry after washing. Add 2.65g of lanthanum chloride to the dried material, the mass of the lanthanum element is 1.5% of the mass of the ternary positive electrode material, then add an appropriate amount of distilled water, stir evenly, form a slurry, and put it into a sagger. Sinter directly at 800° C. for 7 hours in an air atmosphere, cool to room temperature, and grind through a 400-mesh sieve to obtain a lanthanum oxide-coated ternary cathode material.
[0032] The lanthanum oxide-coated LiNi prepared in this example 0.5 co 0.2 mn 0.3 o 2 The positive electrode material was assembled into a battery. The specific method was the same as in Example 1. Under the charge and discharge rate of 0.1C, the initial charge and discharge capacity reached 206 mAh / g and 181 mAh / g, and the initial charge and discharge efficiency was 88%. After 50...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com