A kind of medicine for treating pulmonary infectious disease and application thereof
A technology for pulmonary infection and disease, which is applied to medicines for the treatment of pulmonary infectious diseases and their application fields, and can solve the problems of unclear pharmacological substances and pharmacological effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Example 1: Total flavonoids of Artemisia Sifang or Campanulaceae, neohesperidin dihydrochalcone, naringin dihydrochalcone, 7-methoxywogonin, 6,7-dimethoxybaicalein Primer preparation
[0015] 1) After crushing 5Kg Artemisia sifang or Campanula rhizome, add water 10 times the mass of the medicinal material to extract, concentrate to liquid extract (water content about 12%), add 95% ethanol alcohol 5 times the volume of the extract while it is hot Shen;
[0016] 2) Concentrate the ethanol extract under reduced pressure to a liquid extract (with a water content of about 12%), add distilled water 3 times the volume of the extract to suspend, extract with ethyl acetate 4-5 times, each time the amount is a suspension 3 times the volume of ethyl acetate, decompression or vacuum drying to concentrate the ethyl acetate extract to a dry extract, to obtain the total flavonoids of Artemisia sifanglei or Campanula chinensis;
[0017] 3) Take 20g of total flavonoids of Artemisia qu...
Embodiment 2
[0019] Embodiment 2: Drug efficacy test
[0020] 1 Materials and methods
[0021] Ribavirin effervescent tablets (batch number: 091210), Zhejiang Aikang Lide Pharmaceutical Co., Ltd.
[0022] Influenza A virus mouse lung-adapted strain A / PR / 8 / 34 with a viral titer of 1:512. This experiment was carried out in the ABSL2 biosafety laboratory of Zhejiang Academy of Medical Sciences.
[0023] 1.1 Animals and grouping
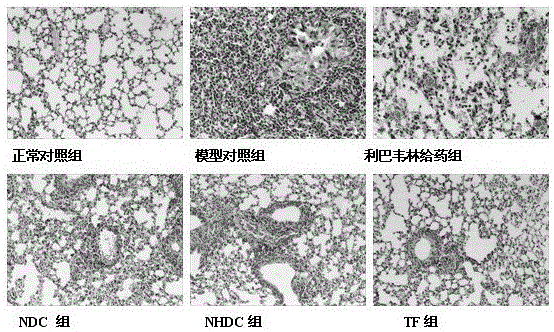
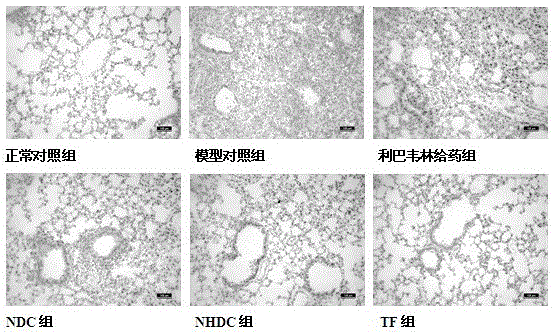
[0024] Clean-grade ICR mice, half male and half male, 18-22 g, provided by Zhejiang Experimental Animal Center, experimental animal license number: SCXK (Zhejiang) 2008-0033. According to body weight, they were randomly divided into 6 groups: normal control group, model control group, ribavirin administration group, neohesperidin dihydrochalcone (NHDC) administration group, naringin dihydrochalcone (NDC) administration group, and naringin dihydrochalcone (NDC) administration group. ) administration group, and the total flavonoids (TF) administration group extract...
Embodiment 3
[0060] Embodiment 3: Drug efficacy test
[0061] 1 Experimental materials
[0062] Clean-grade ICR mice, half male and half male, 18-22 g, provided by Zhejiang Experimental Animal Center, experimental animal license number: SCXK (Zhejiang) 2008-0033.
[0063] Dexamethasone tablets (batch number: 070941), Zhejiang Xianju Pharmaceutical Co., Ltd.; lipopolysaccharide (LPS, batch number: L2880-50MG), American sigma company.
[0064] 2 Experimental methods
[0065] 2.1 Model establishment and grouping
[0066] After adapting to the environment, the mice were randomly divided into 6 groups: normal control group, model group, dexamethasone treatment group, normal control group, model control group, ribavirin administration group and neohesperidin dihydrochalcone (NHDC) administration group, naringin dihydrochalcone (NDC) administration group, total flavonoids (TF) administration group; 10 mice in each group. Continuous intraperitoneal injection for 3 days, 1 time / day, 0.5 hours a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com