Cleaner production method for preparing superfine powder through self-propagating metallurgy method
An ultra-fine powder, clean production technology, applied in the field of metallurgy, can solve the problems of high cost, complex process, high energy consumption, etc., and achieve the effect of high powder activity, high reaction efficiency, and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
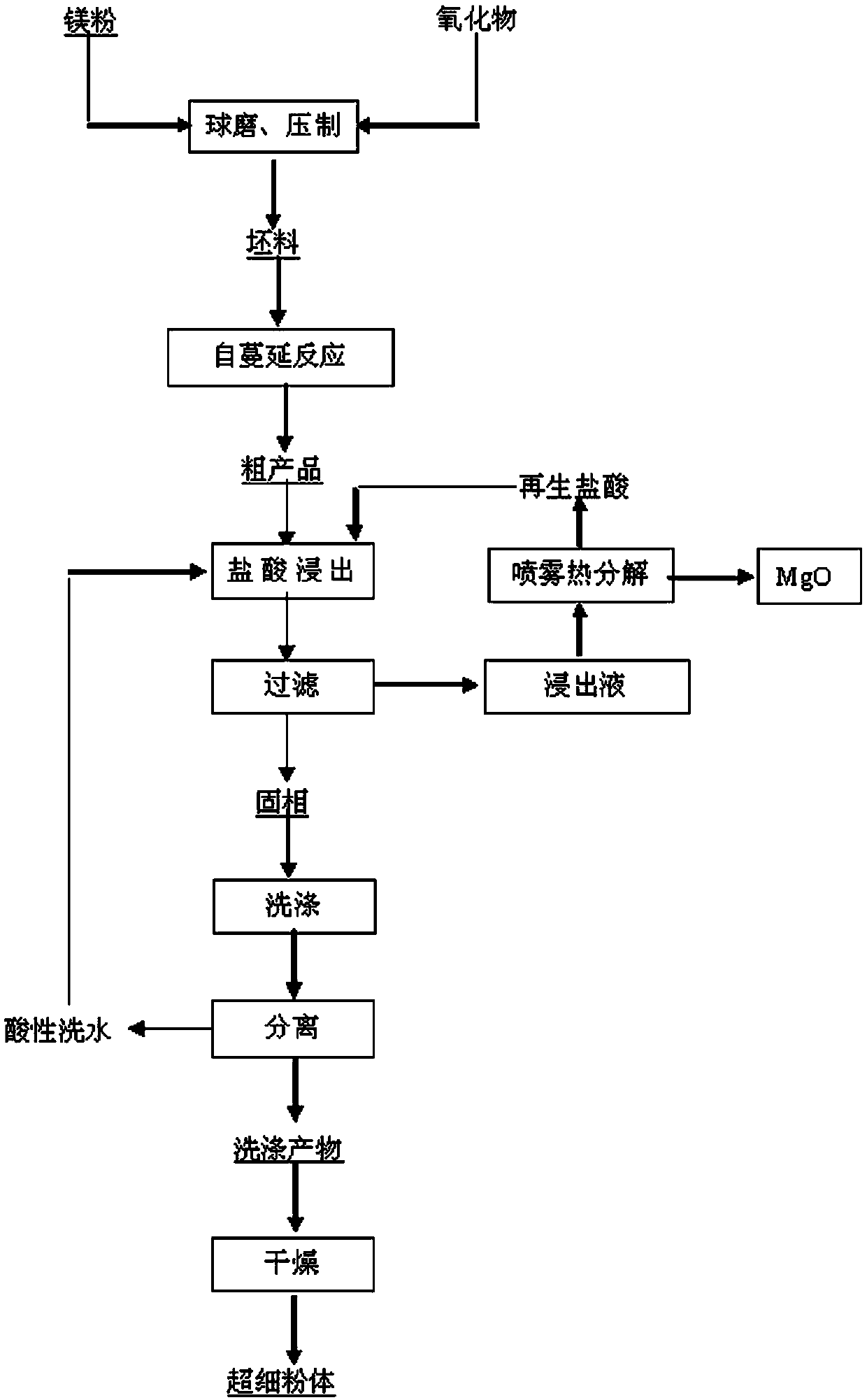
[0040] Mix the powdery boron oxide and magnesium powder and ball mill to a particle size of ≤0.5 μm, then press it into a billet under a pressure of 40 MPa, put it into a self-propagating reaction furnace to initiate a self-propagating reaction; the mixing ratio is boron oxide:magnesium powder by mass =100:110,
[0041] After the self-propagating reaction is finished, the reaction product is naturally cooled to normal temperature to obtain a crude product, and the boron in the crude product is dispersed in the spongy magnesium oxide matrix;
[0042] After the crude product is crushed, the magnesia in it is separated by leaching with hydrochloric acid; the concentration of hydrochloric acid used in leaching is 1mol / L; the liquid-solid ratio of hydrochloric acid to the crude product is 20:1ml / g, and the leaching is carried out at room temperature. The leaching temperature is 90°C and the leaching time is 10h; after leaching, the solid phase and leachate are obtained by filtratio...
Embodiment 2
[0046] Preparation method is with embodiment 1, and difference is:
[0047] (1) Press into a billet under a pressure of 60MPa; the mixing ratio is boron oxide:magnesium powder=100:120 by mass ratio;
[0048](2) The concentration of hydrochloric acid used in the leaching is 3mol / L; the liquid-solid ratio of hydrochloric acid to the crude product is 10:1ml / g; the leaching method is leaching at room temperature, the leaching temperature is 25°C, and the leaching time is 40h; the solid phase is obtained after leaching and leachate; the mass concentration of magnesium chloride in the leachate is 180g / L;
[0049] (3) Spray pyrolysis is to atomize and spray the leaching liquid through the atomizing nozzle into the high-temperature pyrolysis furnace under the pressure of 0.32MPa. The pyrolysis temperature in the high-temperature pyrolysis furnace is 400°C and the pyrolysis time is 10min. ; The particle size of nano-sized magnesium oxide is 80~100nm; the concentration of hydrochloric ...
Embodiment 3
[0051] Preparation method is with embodiment 1, and difference is:
[0052] (1) Press into a billet under a pressure of 70MPa; the mixing ratio is boron oxide:magnesium powder=100:128 by mass ratio;
[0053] (2) The concentration of hydrochloric acid used in the leaching is 5mol / L; the liquid-solid ratio of hydrochloric acid to the crude product is 4:1ml / g; the leaching method is leaching at room temperature, the leaching temperature is 50°C, and the leaching time is 20h; the solid phase is obtained after leaching and leachate; the mass concentration of magnesium chloride in the leachate is 300g / L;
[0054] (3) Spray pyrolysis is to atomize and spray the leaching liquid into the high-temperature pyrolysis furnace through the atomization nozzle under the pressure of 0.6MPa. The pyrolysis temperature in the high-temperature pyrolysis furnace is 200°C and the pyrolysis time is 60min. ; The particle size of nano-sized magnesium oxide is 330~390nm; the concentration of hydrochlori...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com