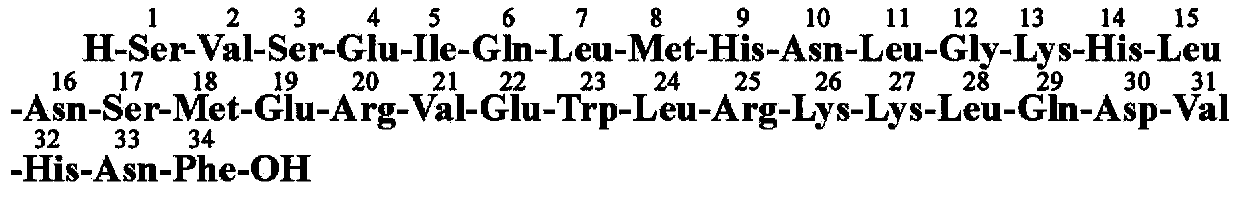Method for preparing teriparatide
A technology of teriparatide and crude peptide, which is applied in the field of medicinal chemistry, can solve the problems of low yield, high price and cost, and cumbersome steps, and achieve the effect of facilitating industrial production, high total yield, and easy price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1: Preparation of Fmoc-Phe-Wang Resin with a degree of substitution of 0.3 mmol / g
[0058] Weigh 625 g (500 mmol) of Wang Resin with a substitution degree of 0.8 mmol / g, add it to a solid-phase reaction column, wash it twice with DMF, and swell the resin with DMF for 30 minutes, take 387.5 g (1000 mmol) of Fmoc-Phe -OH, 162 g (1200 mmol) HOBt, 151.5 g (1200 mmol) DIC, and 12.2 g (100 mmol) DMAP were dissolved in a mixed solution of DCM and DMF with a volume ratio of 1:1, added to a solid-phase reaction column, and reacted at room temperature for 2 Hour. After the reaction, wash 4 times with DMF and 2 times with DCM. Then add 791 g (10000 mmol) pyridine and 1021 g (10000 mmol) acetic anhydride mixed solution to seal the resin for 6 hours. After washing 4 times with DMF and 2 times with DCM, methanol was shrunk and drained to obtain 813.7 g Fmoc-Phe-Wang Resin, and the detection degree of substitution was 0.302 mmol / g (for the method of detection of resin substi...
Embodiment 2
[0059] Embodiment two: the preparation of peptide resin A
[0060] Weigh Fmoc-Phe-Wang Resin331.1g (100mmol) with a substitution degree of 0.302mmol / g, add it to a solid-phase reaction column, wash it twice with DMF, swell the resin with DMF for 30 minutes, and remove the Fmoc protection with DBLK ( Reaction time 5+7 minutes), followed by 4 washes with DMF and 2 washes with DCM. Take 179.0g (300mmol) Fmoc-Asn(Trt)-OH, 48.6g (360mmol) HOBt dissolved in a mixed solution of DCM and DMF with a volume ratio of 1:1, ice bath for 10 minutes, add 45.4g (360mmol) DIC to activate 5 Add it to the solid-phase reaction column after 10 minutes, and react at room temperature for 2 hours (the end point of the reaction is determined by the ninhydrin method. If the resin is colorless and transparent, the reaction is complete, and the color of the resin indicates that the reaction is incomplete, and another coupling reaction is required. 1 h), followed by three washes with DMF.
[0061] Repeat...
Embodiment 3
[0062] Embodiment three: the preparation of peptide resin B
[0063] Take 712.5g of peptide resin A (100.0mmol) prepared in Example 2 and put it into the solid phase reaction column. Take 358.2g (600mmol) Fmoc-Asn(Trt)-OH, 81g (600mmol) HOBt, 7.32g (60mmol) DMAP dissolved in a mixed solution of DCM and DMF with a volume ratio of 1:1, and drop into 75.7 g (600 mmol) DIC, activated for 5 minutes, added to the solid-phase reaction column, and reacted at room temperature for 1 hour. After the reaction, the Fmoc protection was removed with DBLK (reaction time 5+7 minutes), and then washed 4 times with DMF and 2 times with DCM to obtain 778.5 g of peptide resin B.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com