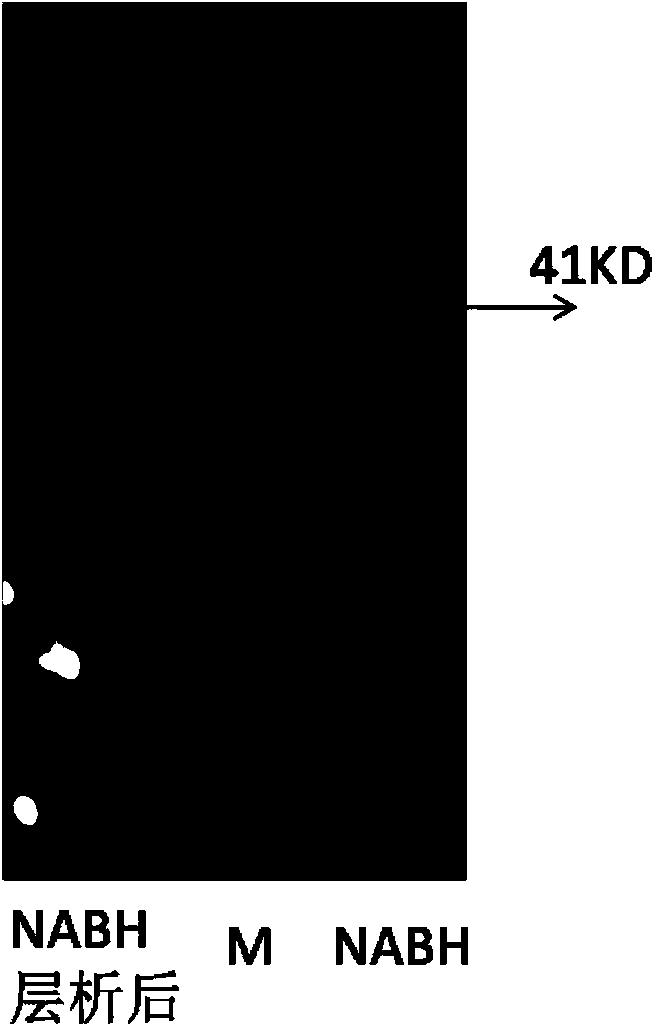
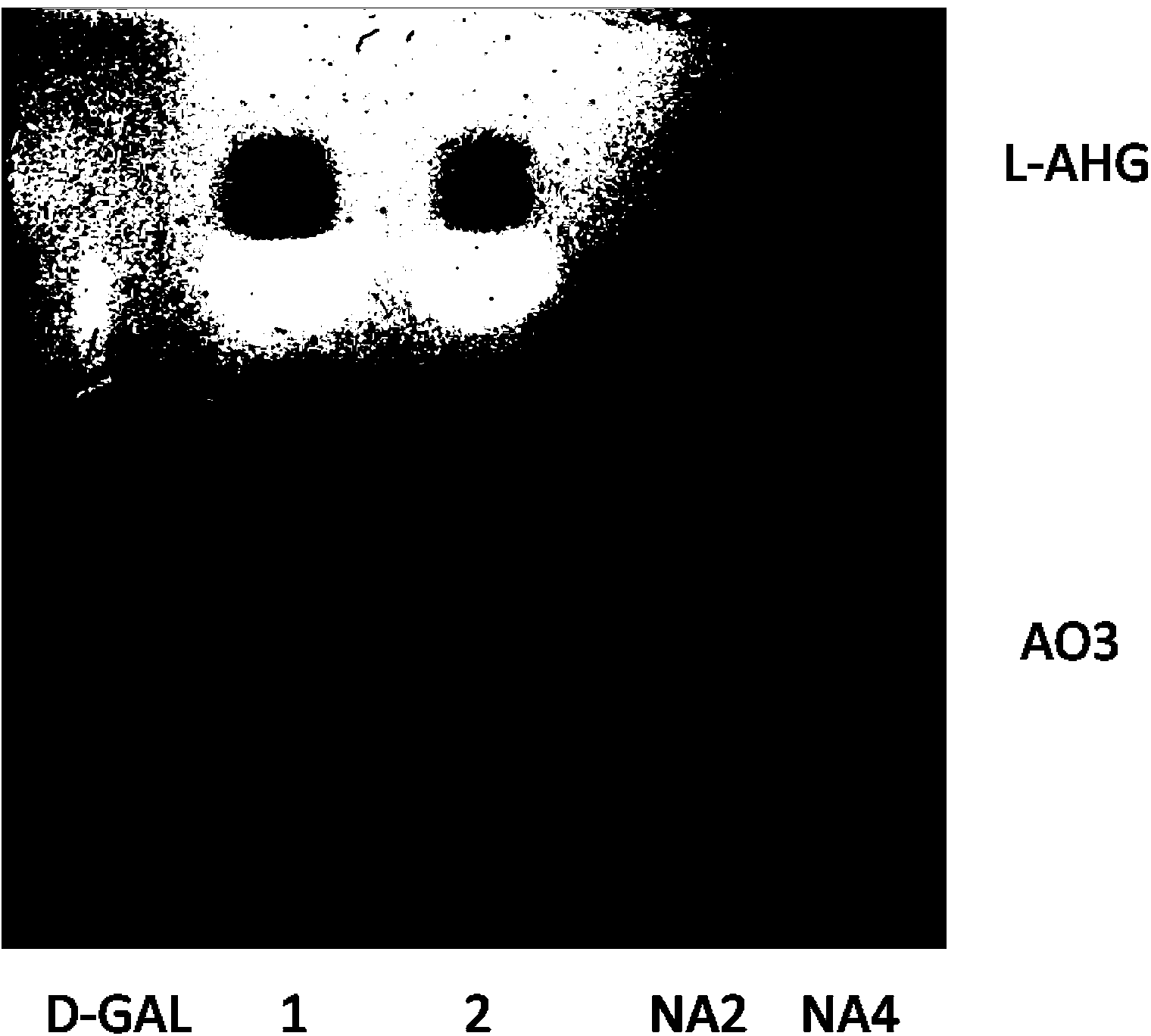
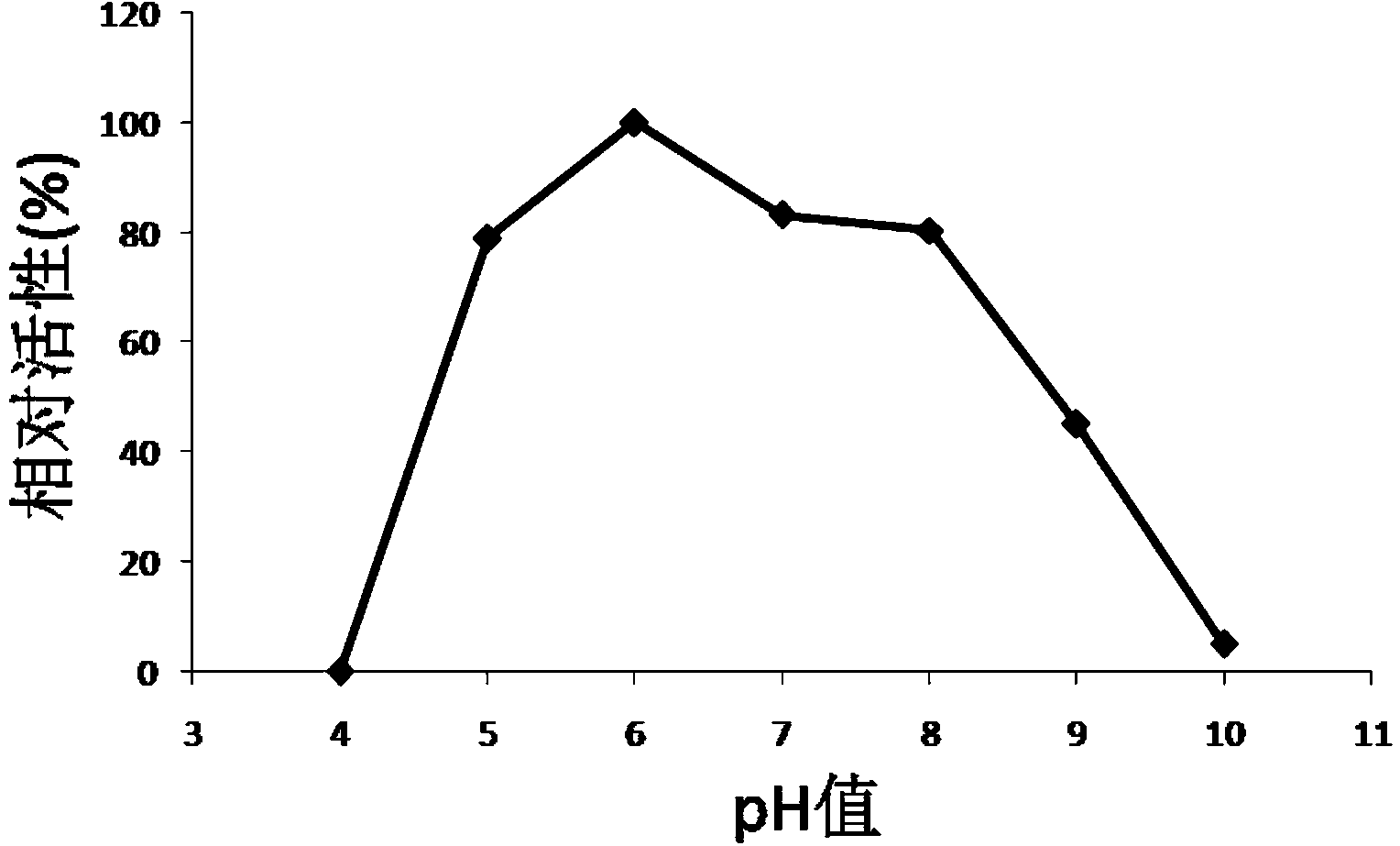
Neoagarobiose hydrolase and application thereof
A new technology of agarobiose and hydrolase, applied in the direction of hydrolase, application, and introduction of foreign genetic material using carriers, etc., can solve the problems of no chemical synthesis reports, etc., and achieve good application potential and prospects, good application value, and reaction Environmentally friendly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Cloning of embodiment 1 new agarobiohydrolase gene
[0019] A new agarobiohydrolase gene from Agarophora chrysanthemum WH0801 (=NRRLB-59247(T)=CGMCC1.10131(T)) was cloned by PCR with degenerate primers and nested PCR. Specific operation: Cultivate Agarophore WH0801 in 2216E seawater medium until the end of the logarithm, and extract the DNA genome. Use specific degenerate primers for amplification. The sequence of the degenerate primers is as follows: 5'-GGYDCSTAYGATGATCGYAGCGTKTTYACC-3'; 5'-GGTRTTTTKTTCCGGRCCATCGGTG-3', using the genome as a template, the conditions are: 94°C for 5 minutes, then 94°C for 30 minutes Seconds, 30 seconds at 55°C, 1 minute at 72°C for 30 cycles, PCR was performed, and the product was a fragment of the new agarobiohydrolase gene, and then primers were designed based on the end sequence of the fragment, and three rounds of nesting were carried out A PCR product fragment with a size of 500Kb-2000Kb was recovered by formula PCR (using the chr...
Embodiment 2
[0020] Embodiment 2 Contains the construction of new agarobiohydrolase gene expression plasmid pETNA
[0021] According to the full-length sequence of the new agarobiohydrolase gene, the upstream primers and downstream primers of the gene were designed (see Example 1), and the genome of Agarophagus pale yellowus WH0801 was used as a template to amplify the new agarobiohydrolase The full-length sequence of the enzyme gene. Conditions were 94°C for 2 minutes, followed by 94°C for 30 seconds, 55°C for 30 seconds, 72°C for 1.5 minutes, and 30 cycles of extension at 72°C for 10 minutes. Agarose gel electrophoresis showed an obvious single band at the position of 1.0kb. The single band was recovered by cutting the gel, and double-digested with Xho1 and BamH1, and the expression vector pET21a was also double-digested with Xho1 and BamH1, and then the PCR product and Restriction vectors are purified using kits. The DNA ligation reaction is then carried out at a certain molar ratio. ...
Embodiment 3
[0022] Example 3 Construction of engineering bacteria pETNA / BL21 highly expressing new agarobiohydrolase gene NABHwh
[0023] The expression vector plasmid pETna was transformed into BL21 (DE3) according to standard calcium chloride heat shock transformation, and positive transformants with ampicillin resistance were screened. The plasmid was extracted by standard alkaline lysis method, and the plasmid was double digested with Xho1 and BamH1, and the gel electrophoresis bands showed two clear bands of 1.0Kb and 5.3Kb, corresponding to the full length of the new agarobiohydrolase gene and the length of the plasmid . It proved that the expression vector plasmid pETNA containing the new agarobiohydrolase gene had been transferred into BL21 (DE3).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com