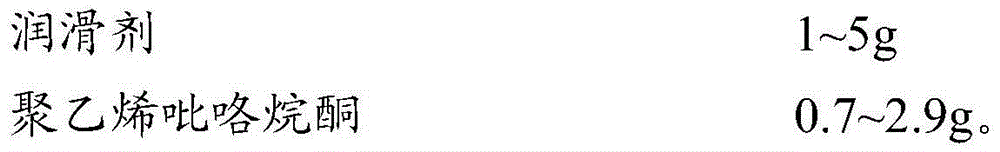Cefixime dispersible tablet and preparation method thereof
A technology of cefixime and dispersible tablets, which is applied in the field of cefixime dispersible tablets and its preparation, can solve the problems of strong hygroscopicity, poor fluidity, and difficulty in popularization and application of granules, and achieve low external pollution, reduced procedures, and low labor intensity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Prescription: based on 1000 tablets
[0037]
[0038] Preparation:
[0039] 1) Adhesive: first add polyvinylpyrrolidone into hot water (80°C-90°C) of 1 / 5-1 / 3 of the total volume, fully disperse and hydrate, then stir continuously under cooling conditions, add Cold water to the total volume, made of 2% polyvinylpyrrolidone aqueous solution, placed in a spray tank for later use.
[0040] 2) Sieving: cefixime is passed through a 100-mesh sieve, and the auxiliary materials are respectively passed through a 80-mesh sieve for later use.
[0041]3) Granulation: Open the lid of the pot, put 50g of cefixime, 10g of pregelatinized starch starch1500, 15g of lactose, 6.5g of low-substituted hydroxypropyl cellulose, 2g of talc, and 1.25g of magnesium stearate into the pot. The one-step granulator is in the feeding car. Turn on the fan and heat it so that the material is evenly heated to 50°C in a fluidized state, turn on the spray button, spray the adhesive, the liquid inlet s...
Embodiment 2
[0045] Prescription: based on 1000 tablets
[0046]
[0047] Preparation:
[0048] 1) Adhesive: first add polyvinylpyrrolidone into hot water (80°C-90°C) of 1 / 5-1 / 3 of the total volume, fully disperse and hydrate, then stir continuously under cooling conditions, add Cold water to the total volume, made of 2% polyvinylpyrrolidone aqueous solution, placed in a spray tank for later use.
[0049] 2) Sieving: cefixime is passed through a 100-mesh sieve, and the auxiliary materials are respectively passed through a 80-mesh sieve for later use.
[0050] 3) Granulation: Open the lid of the pot, and put in the above-mentioned prescription amount of 60g of cefixime, 10g of pregelatinized starch starch1500, 10g of lactose, 6.5g of low-substituted hydroxypropyl cellulose, 2g of talcum powder, and 1.25g of magnesium stearate The one-step granulator is in the feeding car. Turn on the fan and heat it so that the material is evenly heated to 50°C in a fluidized state, turn on the spray ...
Embodiment 3
[0054] Prescription: based on 1000 tablets
[0055]
[0056] Preparation:
[0057] 1) Adhesive: first add polyvinylpyrrolidone into hot water (80°C-90°C) of 1 / 5-1 / 3 of the total volume, fully disperse and hydrate, then stir continuously under cooling conditions, add Cold water to the total volume, made of 2% polyvinylpyrrolidone aqueous solution, placed in a spray tank for later use.
[0058] 2) Sieving: cefixime is passed through a 100-mesh sieve, and the auxiliary materials are respectively passed through a 80-mesh sieve for later use.
[0059] 3) Granulation: Open the lid of the pot, take the above prescription amount of cefixime 40g, pregelatinized starch 1500 20g, lactose 10g, low-substituted hydroxypropyl cellulose 6.5g, talcum powder 2g, magnesium stearate 1.25g Pour into pot. Turn on the fan and heat it so that the material is evenly heated to 50°C in a fluidized state, turn on the spray button, spray the adhesive, the liquid inlet speed is 25-32ml min, the atomi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com