Gene pme16A for encoding metalloprotease and application thereof
A technology of metalloprotease and pme16a, which is applied in application, genetic engineering, plant genetic improvement, etc., to achieve good hydrolysis ability and make up for the shortage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
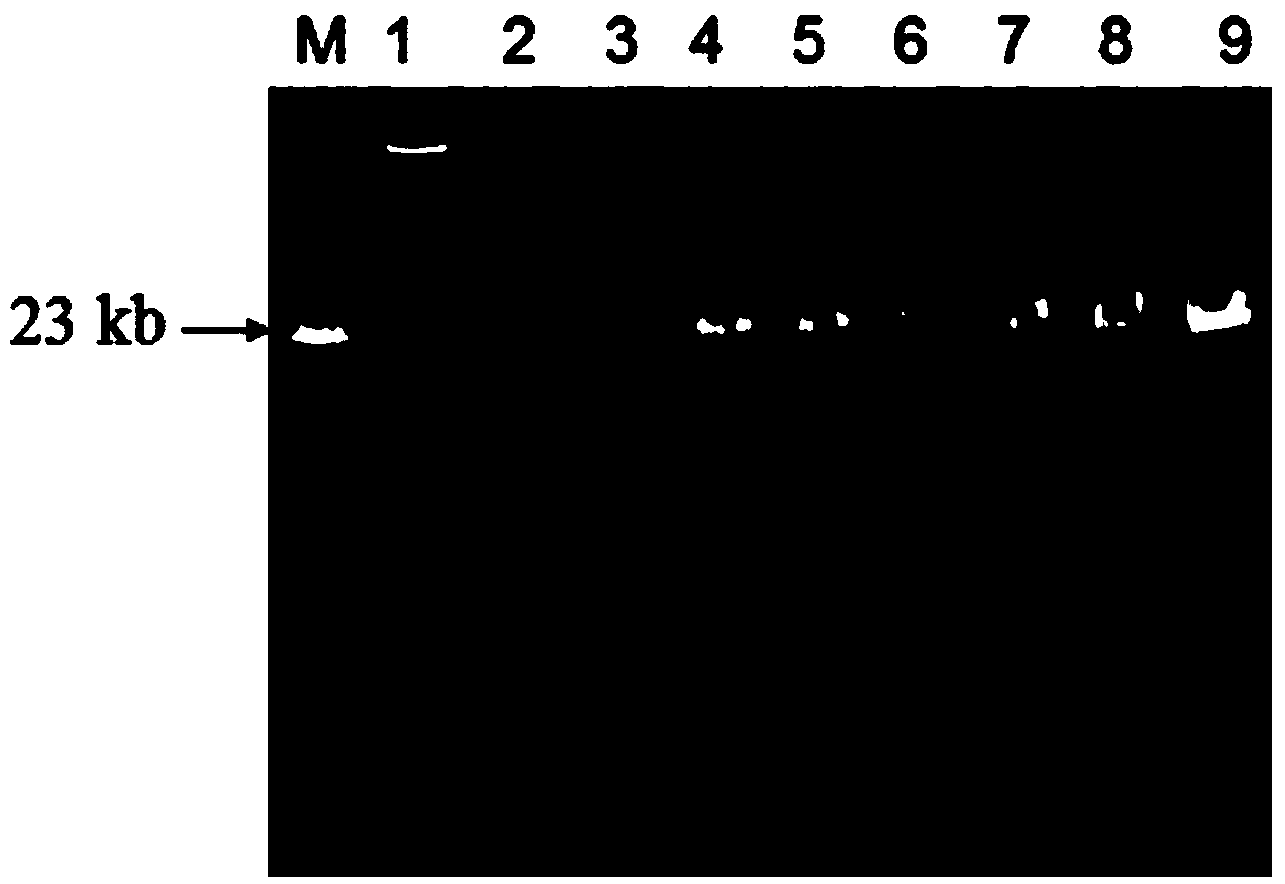
[0028] A clone of gene pme16A encoding novel metalloprotease mainly includes the following steps 1, 2, 3 and 4.
[0029] Step 1: extracting and purifying metagenomic DNA from activated sludge of polluted water samples, the method steps are as follows.
[0030] (1) First prepare the DNA extraction solution: 100mM sodium phosphate buffer (pH8.0), 1% (w / v) CTAB, 100mM EDTA (pH8.0), 0.3M NaCl, 0.01mM Tris-HCl (pH8.0 );
[0031] (2) PVPP pickling treatment: Weigh 10g PVPP, add 3M HCl, soak for 12h, filter with filter paper, wash and stir PVPP with 20mM potassium phosphate buffer (pH7.4), repeat several times until the suspension reaches neutrality, and PVPP filter and dry at 50°C;
[0032] (3) Weigh 1g of sample into a 15mL centrifuge tube, add 0.25g of acidified PVPP, 4mL of extraction buffer, mix well, freeze and thaw at 70°C and liquid nitrogen three times, each time for 30min. After the last dissolution, add SDS to a final concentration of 1%, mix by inversion, place at room...
Embodiment 2
[0052] Construction of pme16A gene expression recombinant plasmid.
[0053] The plasmid pET-32a(+) provided by Novagen, USA was used as the expression vector and E.coli BL21(DE3)pLysS was used as the host cell for heterologous high-efficiency expression of the target gene.
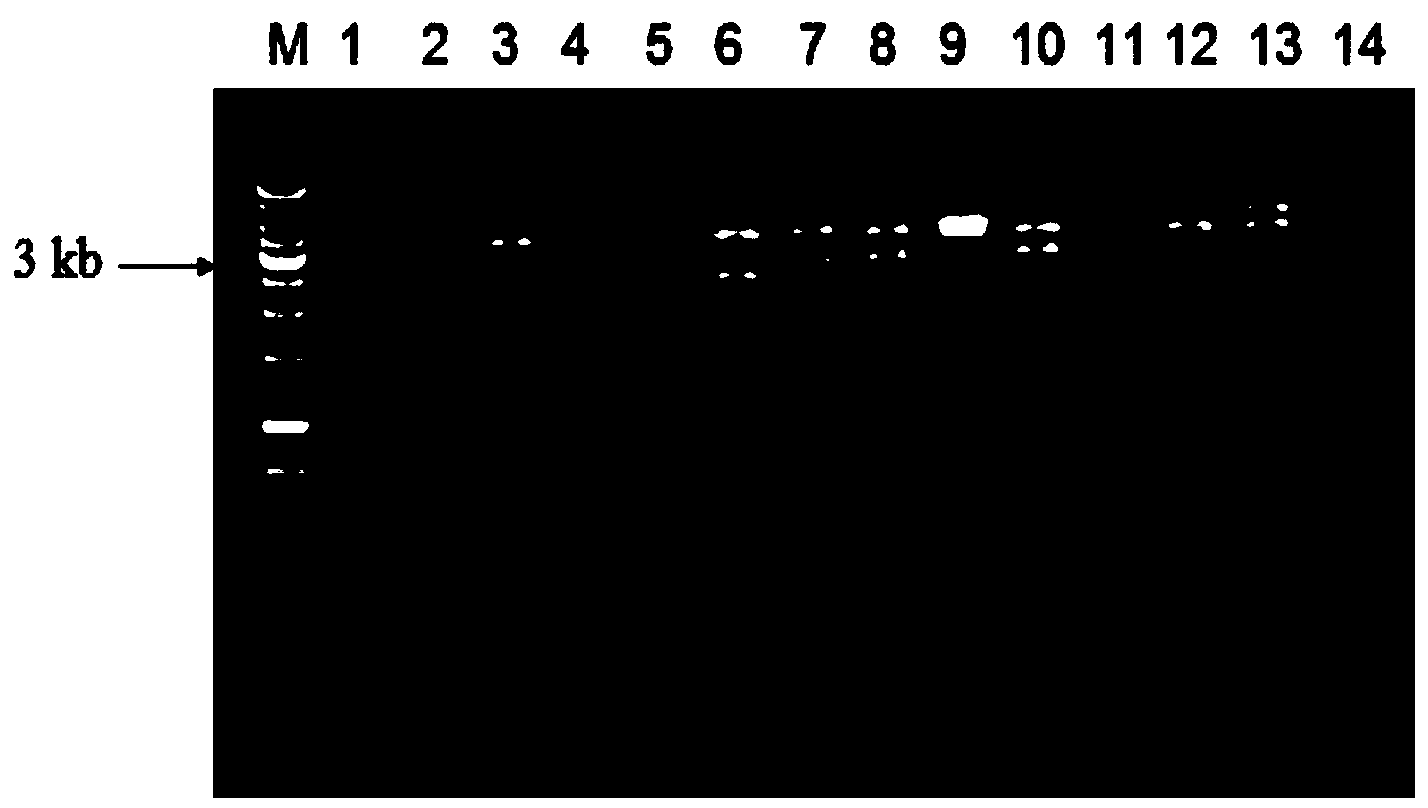
[0054] According to the pme16A gene sequence, primers were designed using related software. Forward amplification primer F1: 5'-GC GAATTC ATGCCCCTTTATCAGACCTTC-3', introduce EcoRI restriction site; reverse amplification primer R1: 5'-AA CTCGAG GGGCGATTCGAGCTC-3', add PstI restriction site. Using pGXM16 as a template, use Pfu DNA polymerase to carry out PCR reaction to amplify the pme16A gene, such as Figure 6 shown. The expression vector pET-32(a)+ plasmid was extracted, digested with EcoRI and PstⅠ, purified and ligated with the pme16A amplification product that had also undergone double digestion and purification, and the expression recombinant plasmid was constructed, and transformed into E .col...
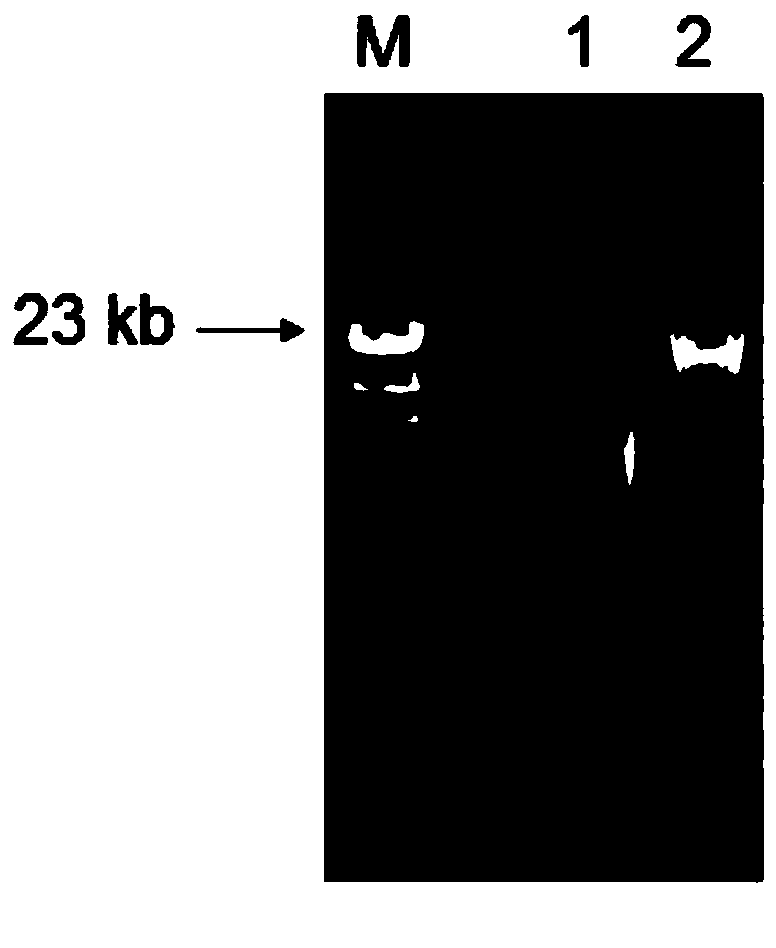
Embodiment 2
[0055] For example two, please refer to Figure 6 . Figure 6To express the test results of recombinant plasmid pGXPME16A, 1 is 1kb Ladder Maker, and the fragment sizes from top to bottom are: 10.0kb, 8.0kb, 6.0kb, 5.0kb, 4.0kb, 3.5kb, 3.0kb, 2.5kb, 2.0kb , 1.5kb, 1.0kb, 750bp, 500bp, 250bp; 2 is the result after digesting pGXPME16A with EcoRI and PstⅠ.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com