Preparation method of carbon-coated tin dioxide superfine powder lithium ion battery negative electrode material
A lithium-ion battery, tin dioxide technology, applied in battery electrodes, circuits, electrical components, etc., can solve the problems of tin dioxide negative electrode capacity, high current discharge capacity, etc., to achieve easy amplification of experimental dose, good high current charge and discharge The effect of stable performance and structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
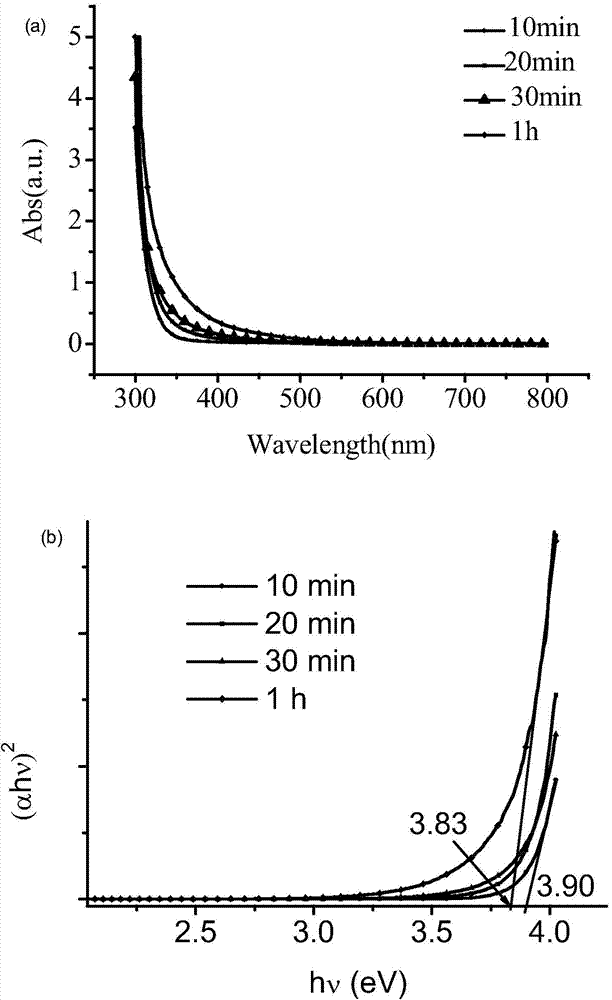
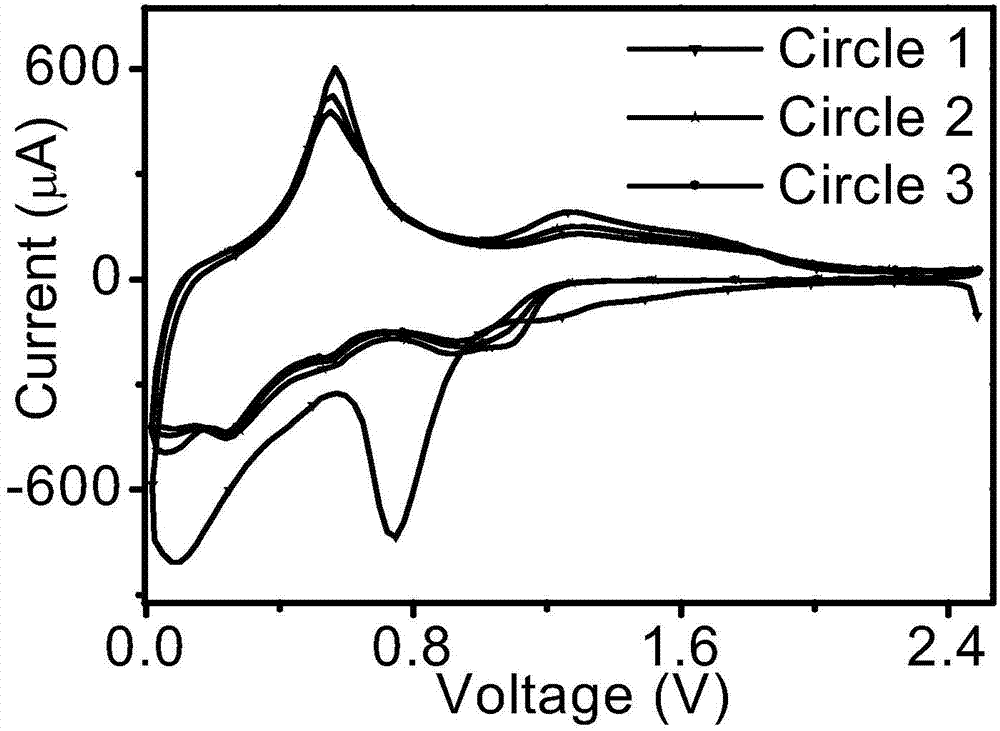
Image
Examples
Embodiment 1
[0030] 1) Mix 0.18g of freshly prepared tin hydroxide hydrate, 2.6ml of oleic acid (OA), 1.0ml of oleylamine (OLA) and 10ml of octadecene (ODE), and vacuumize (0.05mbar) for 30 minutes at room temperature, Heating to 100°C, and then vacuuming for 30 minutes to remove low-boiling impurities; under nitrogen atmosphere, raise the temperature of the reaction solution to 280°C, and then continuously blow air into the reaction solution for 1.5 hours to obtain tin dioxide nanocrystals solution with an air flow rate of 3-4 cm 3 / s;
[0031] 2) Lower the tin dioxide nanocrystal solution to normal temperature, add methanol or isopropanol to precipitate, filter, wash and vacuum dry at 60°C to obtain tin dioxide nanocrystals, and the filtrate is subjected to atmospheric pressure and vacuum fractional distillation for recycling and recycling ;
[0032] 3) Calcining the tin dioxide nanocrystals in a nitrogen atmosphere at 400-600° C. for 3 hours to obtain a carbon-coated tin dioxide powde...
Embodiment 2
[0034] 1) Mix 0.18g of freshly prepared tin hydroxide hydrate, 2.6ml of oleic acid (OA), 1.0ml of oleylamine (OLA) and 10ml of octadecene (ODE), and vacuumize (0.05mbar) for 30 minutes at room temperature, Heating to 100°C, and then evacuating for 30 minutes to remove low-boiling impurities; under a nitrogen atmosphere, raise the temperature of the reaction solution to 250°C, and then continuously blow air into the reaction solution for 1.5 hours to obtain tin dioxide nanocrystals solution with an air flow rate of 3-4 cm 3 / s;
[0035] 2) Lower the tin dioxide nanocrystal solution to normal temperature, add methanol or isopropanol to precipitate, filter, wash and vacuum dry at 60°C to obtain tin dioxide nanocrystals, and the filtrate is subjected to atmospheric pressure and vacuum fractional distillation for recycling and recycling ;
[0036] 3) Calcining the tin dioxide nanocrystals in a nitrogen atmosphere at 600° C. for 3 hours to obtain a carbon-coated tin dioxide powder...
Embodiment 3
[0038] 1) Mix 0.18g of freshly prepared tin hydroxide hydrate, 2.6ml of oleic acid (OA), 1.0ml of oleylamine (OLA) and 10ml of octadecene (ODE), and vacuumize (0.05mbar) for 30 minutes at room temperature, Heat to 100°C, then vacuum for 30 minutes to remove low-boiling impurities; under nitrogen atmosphere, raise the temperature of the reaction solution to 280°C, and then continuously blow air into the reaction solution for 45 minutes to obtain a tin dioxide nanocrystal solution , the air velocity is 3-4cm 3 / s;
[0039] 2) Lower the tin dioxide nanocrystal solution to normal temperature, add methanol or isopropanol to precipitate, filter, wash and vacuum dry at 60°C to obtain tin dioxide nanocrystals, and the filtrate is subjected to atmospheric pressure and vacuum fractional distillation for recycling and recycling ;
[0040] 3) Calcining the tin dioxide nanocrystals in a nitrogen atmosphere at 600° C. for 3 hours to obtain a carbon-coated tin dioxide powder with a size of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com