Method for catalyzing transfer of hydrogen controllable reduction nitro-compound in formic acid or formate
A technology of nitro compounds and formate, applied in the field of fine chemicals, to achieve the effects of high reaction rate, good cycle, high catalytic conversion rate and selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Disperse the semiconductor carbon nitride into deionized water, add an appropriate amount of chloropalladium acid or sodium chloropalladate solution, and soak for a period of time. After adjusting the pH of the chloropalladic acid or sodium chloropalladate solution in which the semiconductor carbon nitride is dispersed with sodium hydroxide solution, adding a reducing agent for reduction, washing, and drying.
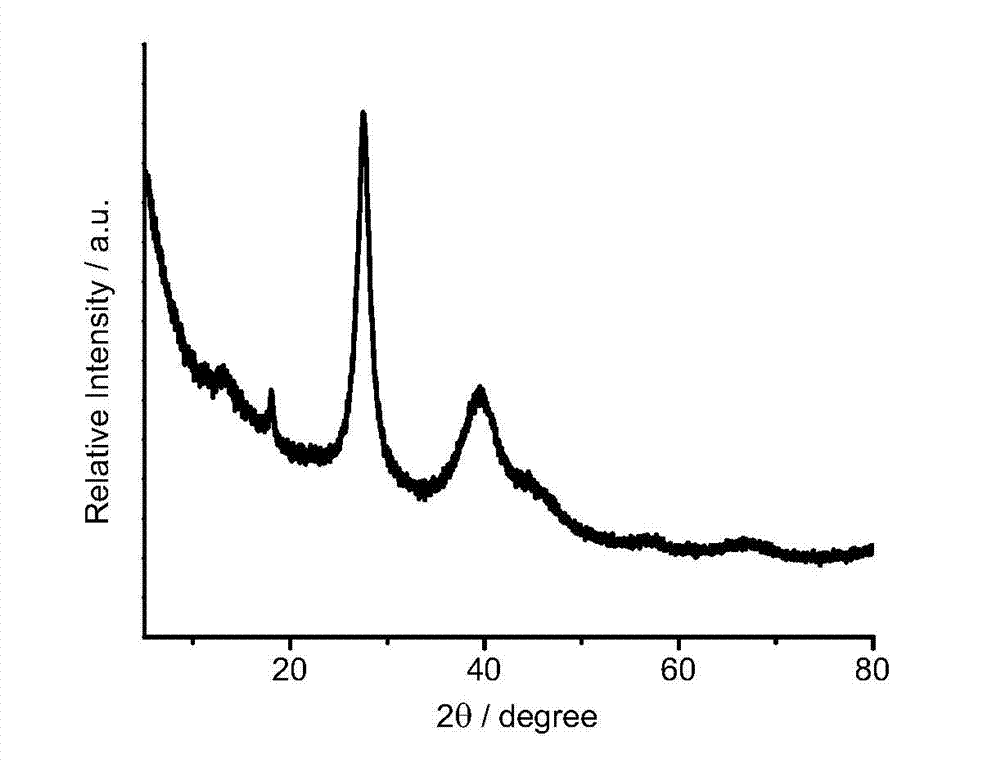
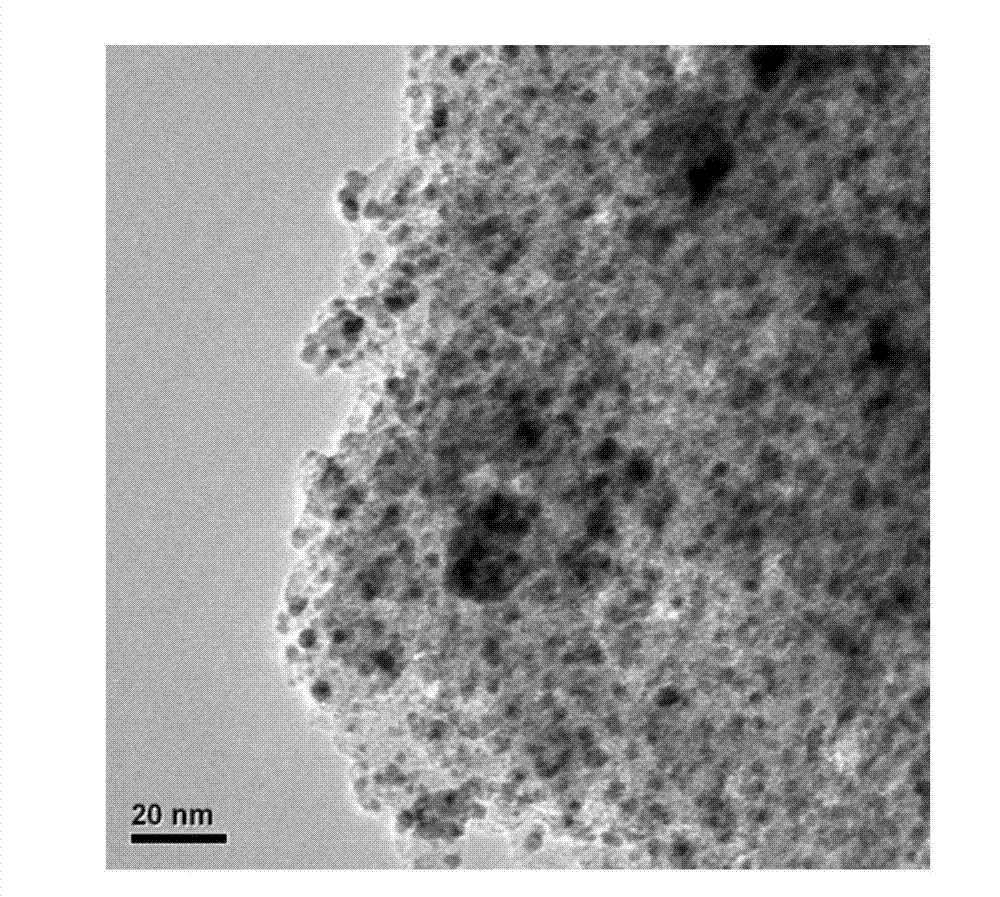
[0026] The chemical composition of the carbon nitride-loaded nano-palladium catalyst prepared by the above method in the present invention is: a palladium-carbon nitride catalyst with a palladium mass fraction of 0.1-40%. figure 1 It is the carbon nitride supported nano-palladium catalyst powder X-ray diffraction spectrogram of embodiment 1, illustrates that there are a large amount of metal palladium (111) and (200) crystal faces to exist; figure 2 It is a transmission electron microscope photo of the carbon nitride-supported nano-palladium catalyst catalyst in...
Embodiment 2
[0028] Disperse polymer semiconductors such as nitrogen-doped graphene, polyacetylene, polyacrylonitrile, poly-p-phenylene acetylene, poly-p-phenylene vinylene, and polythiophene into deionized water, and add appropriate amount of chloropalladium acid or sodium chloropalladate After soaking in the solution for a period of time. After adjusting the pH of the chloropalladic acid or sodium chloropalladate solution in which the polymer semiconductor is dispersed with a sodium hydroxide solution, adding a sodium borohydride solution for reduction, washing, and drying.
[0029] The chemical composition of the polymer semiconductor nano-palladium catalyst prepared by the above method in the present invention is: the polymer semiconductor nano-palladium catalyst with a palladium mass fraction of 0.001:99.999 to 40:60.
Embodiment 3
[0031]Disperse the polymer semiconductor described in Example 2 into deionized water, add an appropriate amount of solutions containing gold, silver, iridium, nickel, ruthenium, platinum, or rhodium, and then soak for a period of time. After adjusting the pH of the solution containing gold, silver, iridium, nickel, ruthenium, platinum or rhodium dispersed with semiconductor carbon nitride with sodium hydroxide solution, adding a reducing agent for reduction, washing and drying. The chemical composition of the polymer semiconductor-supported nano-metal catalyst prepared by the above method in the present invention is: polymer semiconductor-supported gold, silver, iridium, nickel, ruthenium, platinum, or rhodium with a mass fraction of 0.001:99.999 to 40:60 catalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com