Porous silicon material, preparation method and application thereof
A technology of porous silicon and raw materials, applied in chemical instruments and methods, silicon compounds, inorganic chemistry, etc., can solve the problems of complex preparation process, impossibility of industrial production, high equipment requirements, etc., achieve green and clean preparation, avoid toxic solvents or precious metal catalysts The effect of using and prolonging the reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
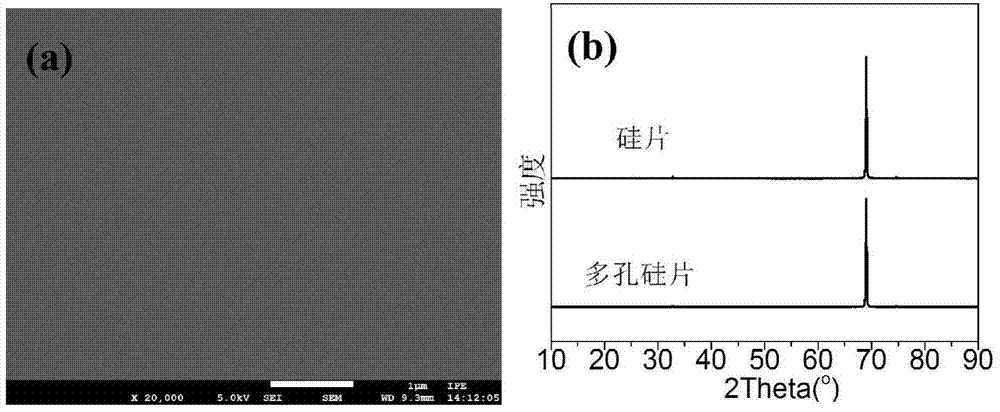
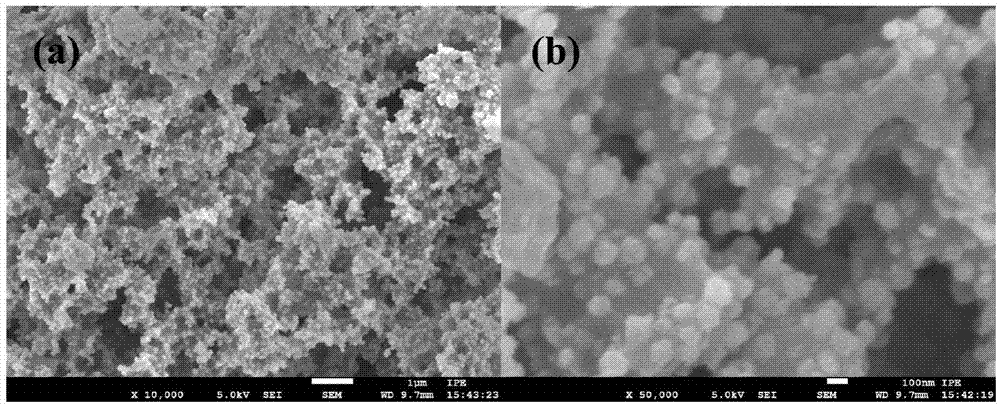
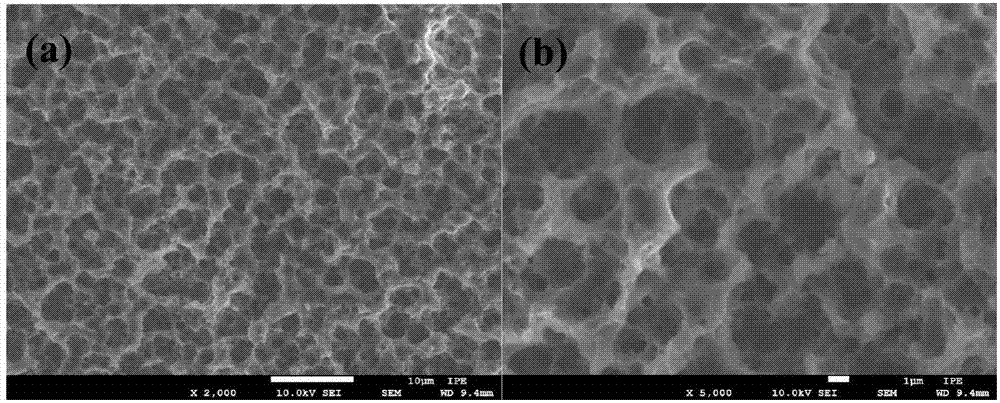
[0050] Disperse commercially purchased silicon wafers, 0.5mmol manganese acetate, 0.5mmol cobalt acetate, 2.0mmol ferric chloride and 15mmol sodium acetate in 80ml of ethylene glycol solution, then put them into a polytetrafluoroethylene-lined reactor, at 200°C , after 48 hours of reaction, cooled to room temperature, the Mn produced during the reaction 0.5 co 0.5 Fe 2 o 4 The powder can be recycled after being separated from ethylene glycol. After the reaction, the silicon chip containing the transition metal oxide catalyst was washed 5 times with deionized water, ultrasonically treated in hydrochloric acid at 90°C for 2 hours to remove the catalyst on the surface of the silicon chip, washed 5 times repeatedly with deionized water, and then placed in The reaction was stirred in sodium hydroxide solution for 24 hours to remove the silicon dioxide on the surface of the porous silicon, washed repeatedly with deionized water for 5 times, and dried in a vacuum oven at 80°C for ...
Embodiment 2
[0057] Disperse commercially purchased silicon wafers, 0.4mmol zinc acetate, 1.6mmol cobalt acetate, 4.0mmol ferric chloride and 10mmol sodium acetate in 80ml of ethylene glycol solution, then put them into a polytetrafluoroethylene-lined reactor, at 200°C , after 62 hours of reaction, cooled to room temperature, the Zn generated during the reaction 0.2 co 0.8 Fe 2 o 4 The powder can be recycled after being separated from ethylene glycol. After the reaction, the silicon chip containing the transition metal oxide catalyst was washed 5 times with deionized water, ultrasonically treated in hydrochloric acid at 90°C for 2 hours to remove the catalyst on the surface of the silicon chip, washed 5 times repeatedly with deionized water, and then placed in The reaction was stirred in sodium hydroxide solution for 24 hours to remove the silicon dioxide on the surface of the porous silicon, washed repeatedly with deionized water for 5 times, and dried in a vacuum oven at 80°C for 24 h...
Embodiment 3
[0061] Commercially purchased silicon wafers, 2.0mmol cobalt acetate, 4.0mmol ferric chloride and 12mmol sodium acetate were dispersed in a mixture solution of 60ml ethylene glycol and 20ml ethanol, then loaded into a polytetrafluoroethylene-lined reactor, at a temperature of 250°C, After reacting for 80 hours, cool to room temperature, the CoFe generated in the reaction process 2 o 4 The powder can be recycled after being separated from ethylene glycol and ethanol. After the reaction, the silicon chip containing the transition metal oxide catalyst was washed 5 times with deionized water, ultrasonically treated in hydrochloric acid at 90°C for 2 hours to remove the catalyst on the surface of the silicon chip, washed 5 times repeatedly with deionized water, and then placed in The reaction was stirred in sodium hydroxide solution for 24 hours to remove the silicon dioxide on the surface of the porous silicon, washed repeatedly with deionized water for 5 times, and dried in a va...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com