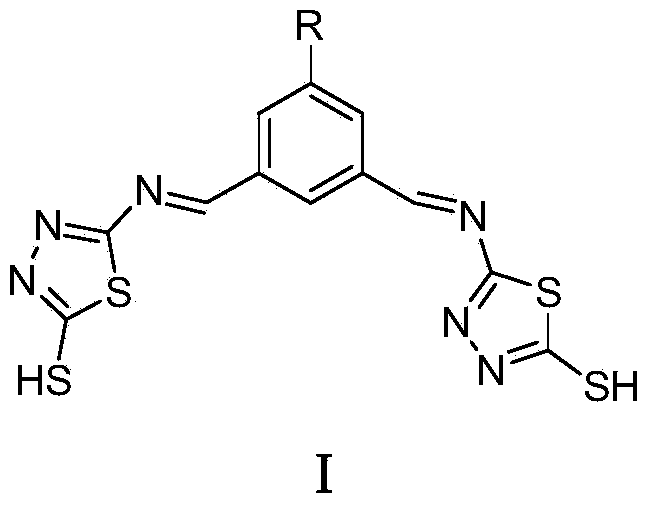
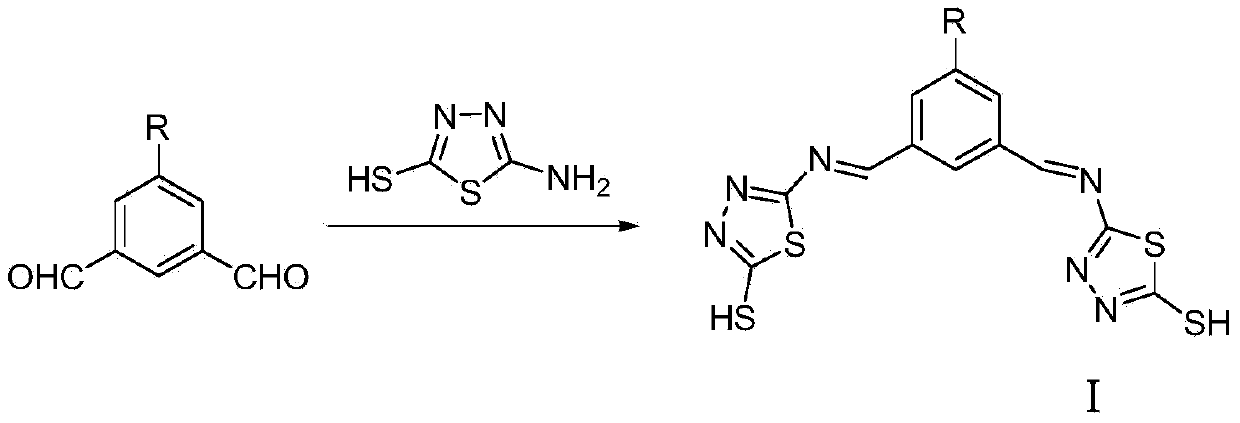
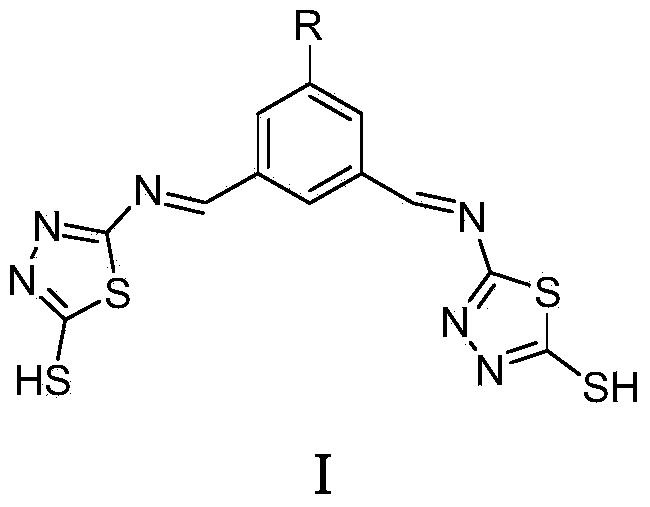
2-mercapto-1,3,4-thiadiazole derivative, and preparation method and application thereof
A technology of thiadiazole derivatives and thiadiazoles, applied in the directions of botanical equipment and methods, applications, biocides, etc., to achieve the effects of mild conditions, broad-spectrum antibacterial activity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Synthesis of N,N'-bis((2'-mercapto-1',3',4'-thiadiazole)-5')-5-nitro-1,3-dimethylaminobenzene
[0029] 2-Mercapto-5-amino-1,3,4-thiadiazole (2mmol, 266mg) was dissolved in absolute ethanol (20mL), and 3 drops of glacial acetic acid were added under magnetic stirring, 5-nitro-1,3- Phthalaldehyde (1 mmol, 179 mg) was added dropwise to the above solution. In a nitrogen atmosphere, the mixture was heated to reflux for 12 h. After the reaction was completed, cooled and filtered with suction to obtain a yellow target product, which was recrystallized from absolute ethanol, washed with ether, and dried in vacuo to obtain a yellow solid. Yield: 87%. 1 H NMR (400MHz, DMSO-d 6 , 298K) δ13.17 (s, 2H, SH), 10.64 (s, 2H, CH), 7.09 (s, 3H, ph-H). Elemental Analysis: Calculated Value C 12 h 7 N 7 o 2 S 4 : C, 35.20; H, 1.72; N, 23.94; Experimental values: C, 35.51; H, 1.29; N, 24.18. ESI-MS(m / z):407.8(M-H) - .
Embodiment 2
[0031] UV-Vis Absorption Spectroscopy Experiment
[0032] The compound N,N'-bis((2'-mercapto-1',3',4'-thiadiazole)-5')-5-nitro-1,3-dimethylamine synthesized by the present invention Base benzene is the main body dubbed 1×10 -3 mol / L dimethyl sulfoxide solution, the anion tetrabutylammonium salts are tetrabutylammonium fluoride, tetrabutylammonium chloride, tetrabutylammonium bromide, tetrabutylammonium iodide, tetrabutylammonium Ammonium acetate and tetrabutyl ammonium dihydrogen phosphate are both made into 2×10 -3 mol / L dimethyl sulfoxide solution. Pipette 0.2mL of the main body solution into a series of 5mL colorimetric tubes, add a certain volume of anion tetrabutylammonium salt solution to each colorimetric tube, and then dilute to the mark with dimethyl sulfoxide solvent to obtain a series of main body For solutions with constant concentration and different anion concentrations, the absorption spectrum is measured after mixing evenly, and the dimethyl sulfoxide solven...
Embodiment 3
[0039] Fluorescence emission spectroscopy experiments
[0040] The compound N,N'-bis((2'-mercapto-1',3',4'-thiadiazole)-5')-5-nitro-1,3-dimethylamine synthesized by the present invention Base benzene is the main body dubbed 1×10 -3 mol / L dimethyl sulfoxide solution, the anion tetrabutylammonium salts are tetrabutylammonium fluoride, tetrabutylammonium chloride, tetrabutylammonium bromide, tetrabutylammonium iodide, tetrabutylammonium Ammonium acetate and tetrabutyl ammonium dihydrogen phosphate are both made into 2×10 -3 mol / L dimethyl sulfoxide solution. Pipette 0.2mL of the main body solution into a series of 5mL colorimetric tubes, add a certain volume of anionic tetrabutylammonium salt solution to each colorimetric tube, and then dilute to the mark with dimethyl sulfoxide to obtain a series of compound concentrations For solutions with constant and different anion concentrations, measure their fluorescence emission spectra after mixing evenly, and use dimethyl sulfoxide...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com