Novel niacin compound slow-release preparation for treating hyperlipoidemia
A slow-release preparation, hyperlipidemia technology, applied in pharmaceutical formulations, metabolic diseases, ester active ingredients, etc., can solve problems such as large toxic and side effects, and achieve the effects of reduced toxic and side effects, good therapeutic effect, and reduced content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Preparation of Niacin (300mg) Lovastatin (6mg) Sustained Release Capsules
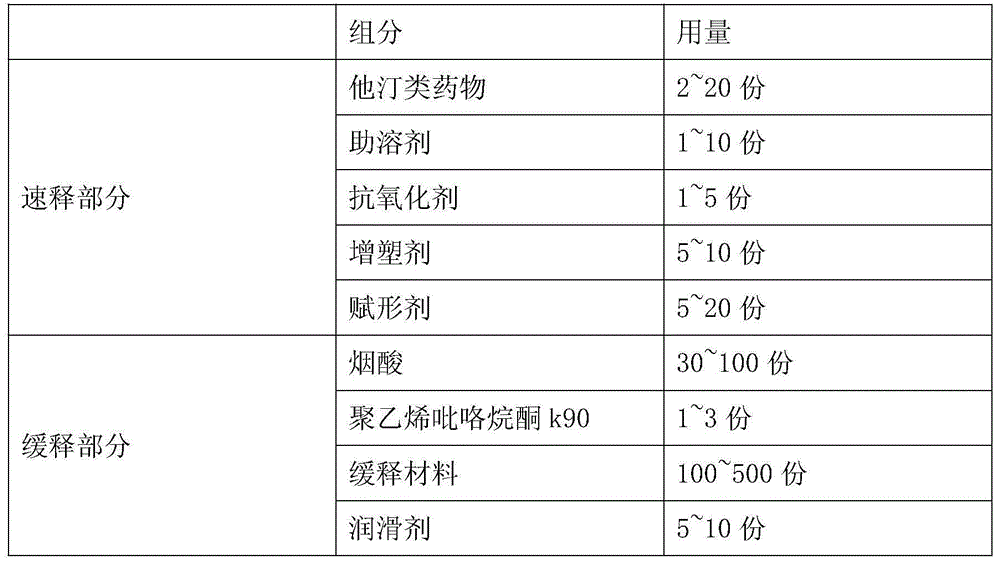
[0038] Niacin Extended Release Part A:
[0039]
[0040]Lovastatin immediate release part B:
[0041]
[0042] Preparation A of niacin sustained-release pellets: Weigh the prescribed amount of niacin, polyvinylpyrrolidone k90, microcrystalline cellulose, ethyl cellulose, and stearic acid, first pass niacin through a 60-mesh sieve, and then niacin Put it into a wet granulator together with auxiliary materials, add an appropriate amount of purified water to make soft materials, use an extrusion spheronizer to make pellets, dry, and screen 18-30 pellets to obtain niacin sustained-release pellets.
[0043] Preparation B of lovastatin immediate-release pellets: Weigh the prescribed amount of lovastatin, starch, microcrystalline cellulose, dextrin, and lactose, add lovastatin and auxiliary materials to the wet granulator and add appropriate amount of purified water Prepare soft mate...
Embodiment 2
[0046] Example 2: Preparation of Niacin (1200mg) Lovastatin (14mg) Double Layer Tablets
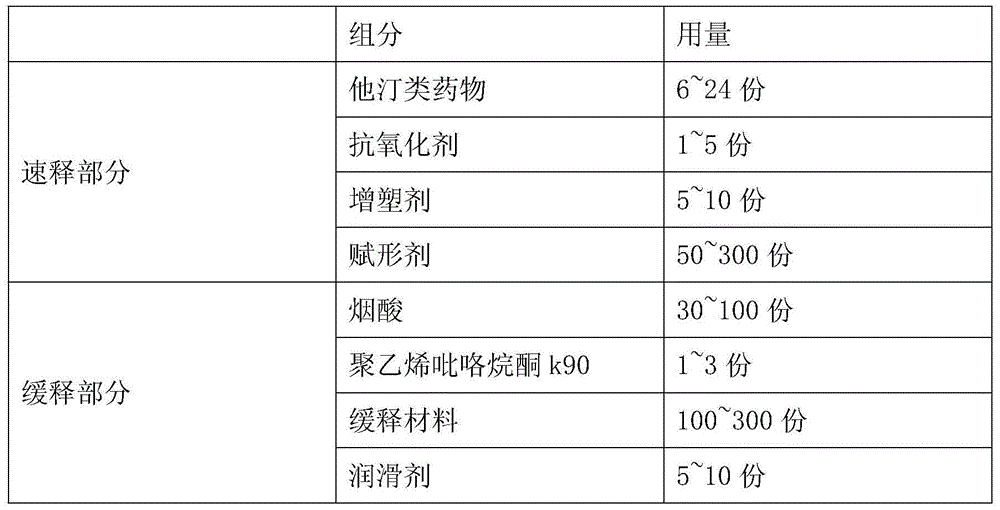
[0047] Niacin Extended Release Part A:
[0048]
[0049]
[0050] Simvastatin Part B:
[0051]
[0052] Part A of Niacin Sustained-release Tablets: Weigh the prescribed amount of Niacin, Polyvinylpyrrolidone K90, and Hypromellose (K15M) added inside. First pass the niacin through a 60-mesh sieve, and then add the niacin and internal auxiliary materials into a wet granulator for granulation. The prepared soft material is wet sized, dried in a fluidized bed, sized, and weighed to calculate the yield. Weigh the added hypromellose (K15M) and stearic acid, mix and set aside.
[0053] Simvastatin immediate-release part B: weigh the prescribed amount of simvastatin, microcrystalline cellulose, pregelatinized starch, lactose, and stearic acid. Use purified water as a wetting agent, manually granulate, wet granules with 16 mesh for soft materials, dry in an oven at 50-55 for 2-5 hours...
Embodiment 3
[0056] Example 3: Preparation of Niacin (800mg) Lovastatin (16mg) Double Layer Tablets
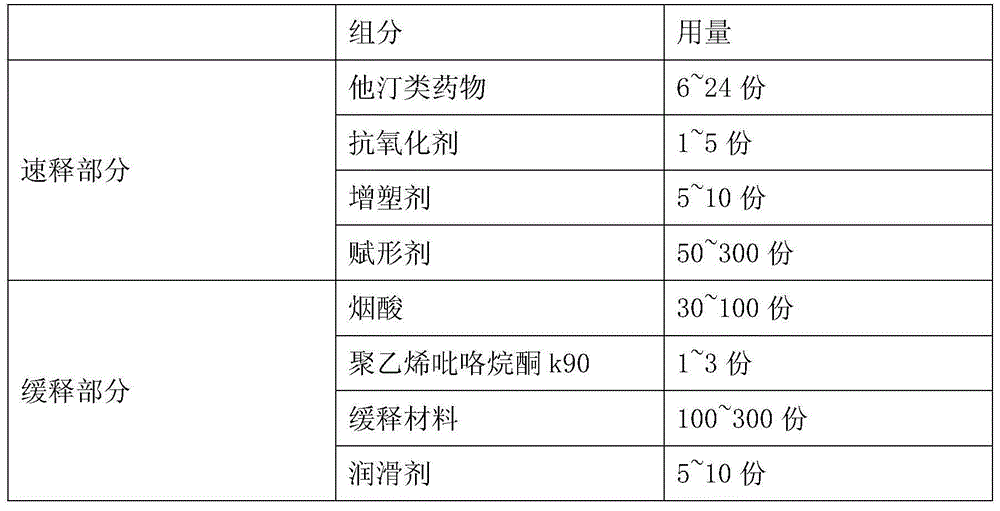
[0057] Niacin Extended Release Part A:
[0058]
[0059]
[0060] Simvastatin Part B:
[0061]
[0062] Part A of Niacin Sustained-release Tablets: Weigh the prescribed amount of Niacin, Polyvinylpyrrolidone K90, and Hypromellose (K15M) added inside. First pass the niacin through a 60-mesh sieve, and then add the niacin and internal auxiliary materials into a wet granulator for granulation. The prepared soft material is wet sized, dried in a fluidized bed, sized, and weighed to calculate the yield. Weigh the added hypromellose (K15M) and stearic acid, mix and set aside.
[0063] Simvastatin immediate-release part B: weigh the prescribed amount of simvastatin, microcrystalline cellulose, pregelatinized starch, lactose, and stearic acid. Use purified water as a wetting agent, manually granulate, wet granules with 16 mesh for soft materials, dry in an oven at 50-55 for 2-5 hours,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com