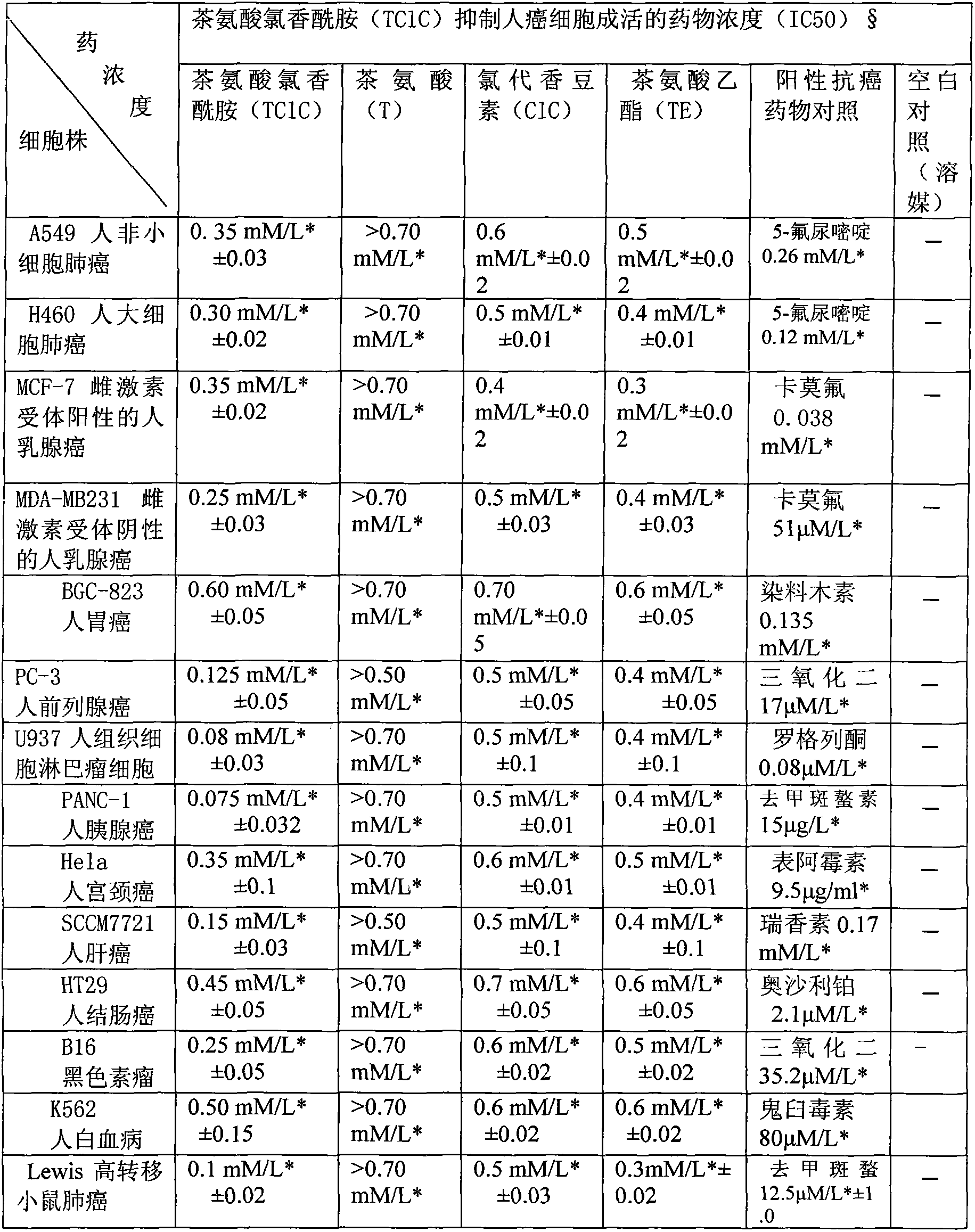
Application of ethyl 6-chlorocoumarin-3-carboxylyl L-theanine and the like in preparation of product used for preventing and treating disease such as cancers
The technology of theanine chloroaromatic amide and theanine ethyl ester is applied in the medical field, can solve the problems of toxic and side effects, limited prevention and treatment, and achieves the effects of less toxic and side effects, enhanced curative effect and wide application.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
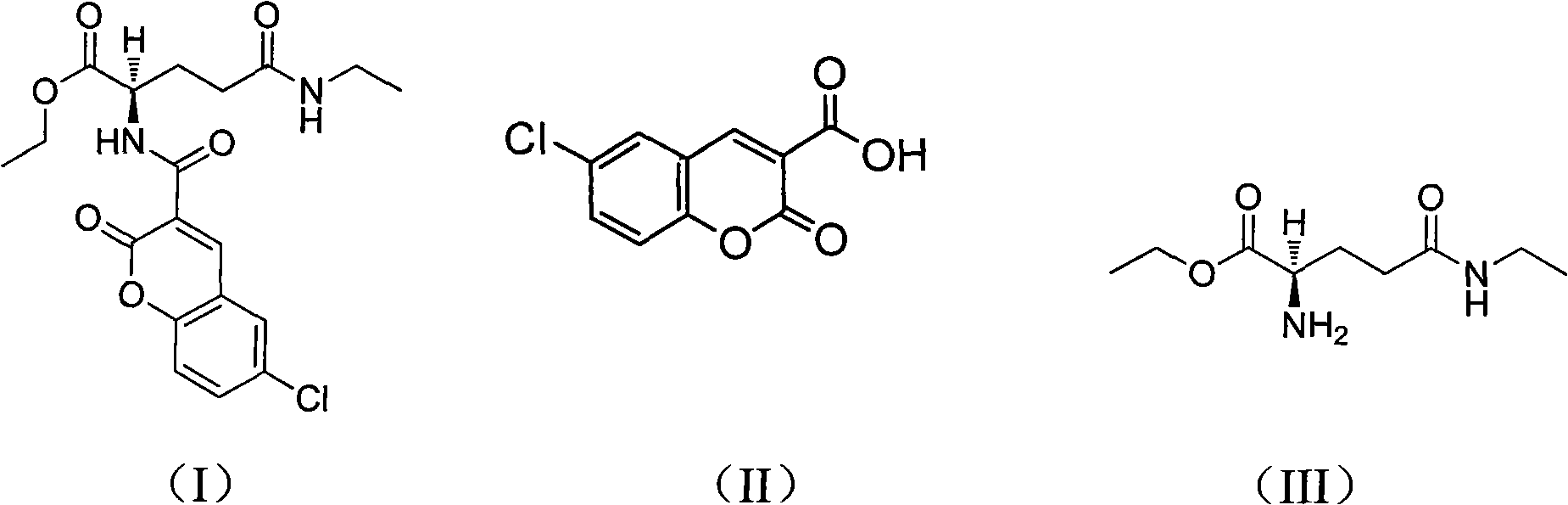
[0038] A compound for treating tumors in this embodiment, its name is (R)-ethyl ester-2-(6-Cl-2-O-2H-benzopyran-3-carboxy)-5-(ethylamino) -5-oxopentanoic acid ester, referred to as theanine chloroaromatic amide (TClC), has the chemical structural formula shown in formula (I):
[0039] As the compound shown in formula (I), the physical properties are light yellow powdery solid, and the melting point decomposes above 242 ° C;
[0040] The compound shown in formula (I) is a pharmaceutically acceptable carrier; the pharmaceutically acceptable carrier includes any substance suitable for injection, preferably water for injection, or emulsifier, liposome, nano preparation and other pharmaceutical carriers.
[0041] The preparation method of compound theanine chloro-aromatic amide as shown in formula (I), mainly comprises the following steps:
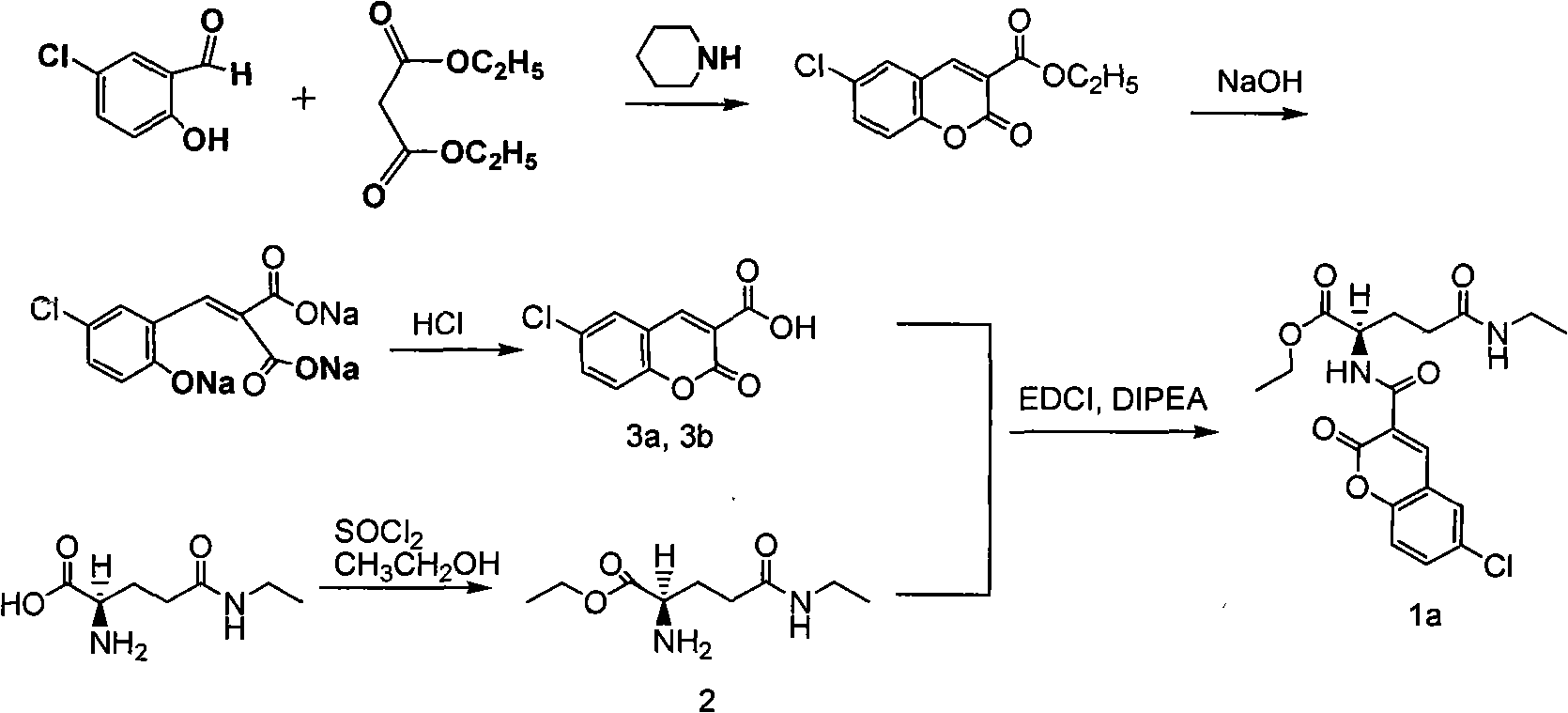
[0042] 1, the preparation of intermediate 6-chlorocoumarin-3-carboxylic acid, as shown in structural formula (II);
[0043] 2, the preparat...
Embodiment 2
[0053] The difference between this embodiment and embodiment 1 is:
[0054] Step 1: Preparation of 6-chloro-coumarin-3-carboxylic acid
[0055] (1) Add 205g of 5-chlorosalicylaldehyde, 205mL of diethyl malonate, 610mL of absolute ethanol, 11mL of hexahydropyridine and 1-1.3ml of glacial acetic acid; (3) Add about 610mL of cold water (0°C), filter with suction after crystallization and wash twice with 110mL of 50% ethanol cooled by ice water (0°C), to obtain 6- Chlorocoumarin-3-condensed ester; (4) add 128g of 6-chlorocoumarin-3-carboxylate ethyl ester and 103g sodium hydroxide respectively, then add 510ml absolute ethanol and 510mL water, 80 ℃ water bath Under certain conditions, heat to reflux for about 2.5 hours; (5) After the reaction is completed, immediately place it in an ice bath at 0°C, add concentrated hydrochloric acid (HCL content: 36-38%), and make the pH of the system between 2-3, and the system will The solid precipitated, was filtered after cooling in an ice b...
Embodiment 3
[0061] The difference between this embodiment and embodiment 2 is that
[0062] Step 1: Preparation of 6-chloro-coumarin-3-carboxylic acid
[0063] (1) Add 210g of 5-chlorosalicylaldehyde, 210mL of diethyl malonate, 615mL of absolute ethanol, 12mL of hexahydropyridine and 1-1.3ml of glacial acetic acid; (3) Add about 615mL of cold water (0°C), filter with suction after crystallization and wash twice with 115mL of 50% ethanol cooled by ice water (0°C), to obtain 6-chloro Coumarin-3-condensed ester; (4) Add 132g and 106g sodium hydroxide respectively, then add 515ml absolute ethanol and 515mL water, 80 ℃ water bath conditions (5) Immediately after the reaction is completed, put it in an ice bath at 0°C, add concentrated hydrochloric acid (HCL content: 36-38%), and make the pH of the system between 2-3, and the system will precipitate solids , filtered after cooling in an ice bath, washed with a small amount of ice water, and the dried crude product was purified by recrystalliz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com