Method for preparing carboxyl polystyrene copolymerized fluorescent microsphere
A technology of fluorescent microspheres and polystyrene, applied in chemical instruments and methods, luminescent materials, etc., can solve the problems of destroying polymerization conditions and difficult microspheres, and achieve high luminous efficiency, low cost, and uniform particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
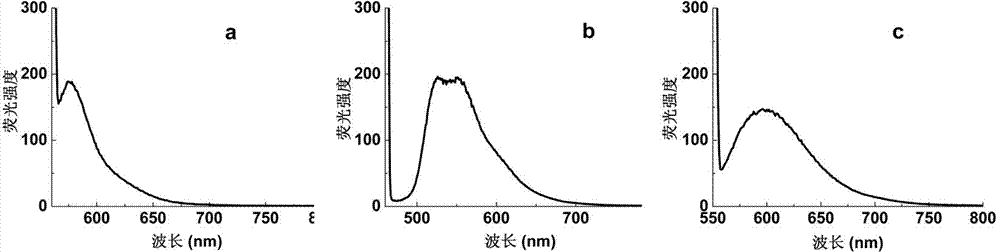
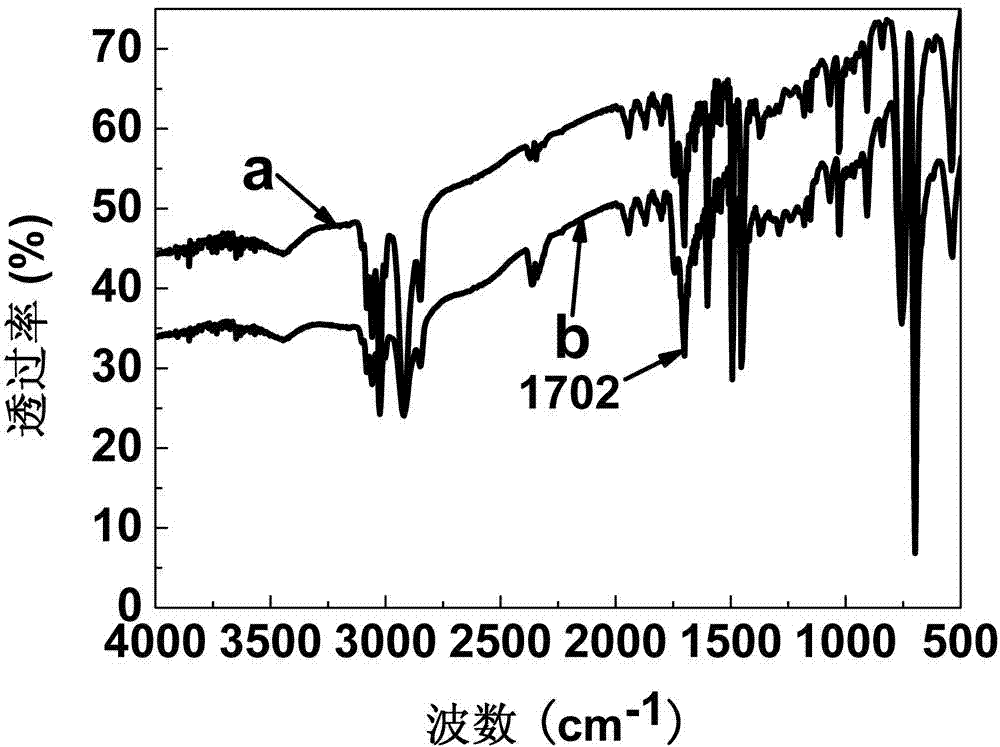
[0018] 1. Synthesis of allyl rhodamine B: According to the literature (J. Mater. Chem., 2009, 19, 2018-2025) method, put 240g of rhodamine B, 0.73g of potassium carbonate, and 2.65g of 3-bromopropene into the reaction In the bottle, add solvent DMF 50ml, catalyst iodine trace, polymerization inhibitor hydroquinone trace, under anhydrous and oxygen-free conditions, react at 71°C for 25h to synthesize allyl rhodamine B, and the crude product is obtained by column chromatography Pure.
[0019]
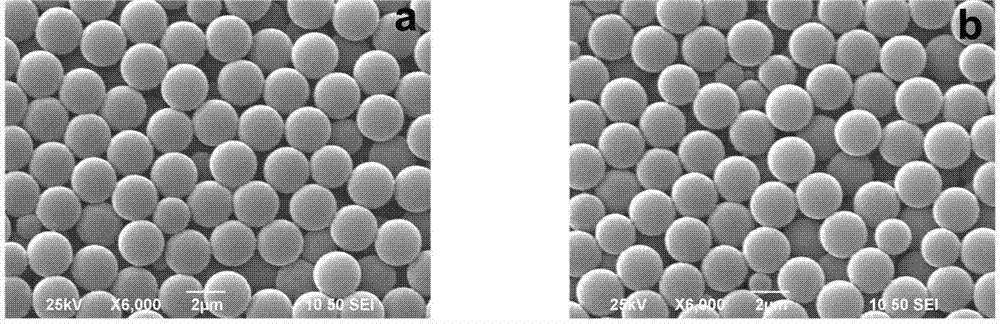
[0020] 2. Preparation of carboxypolystyrene copolymerized fluorescent microspheres: take 0.06g of azobisisobutyronitrile, 0.18g of polyvinylpyrrolidone, 9.6ml of absolute ethanol and 0.4ml of water respectively, put them in a microreactor, and ultrasonically clarify Clear solution. Add 3.02 g of styrene, 0.15 g of acrylic acid, and 15 mg of allyl rhodamine B, pass nitrogen to remove oxygen, and seal. Place in a constant temperature oscillator and react at 70°C for 12h. The obtained ...
Embodiment 2
[0024] 1. Synthesis of allyl fluorescein: According to the literature (J. Mater. Chem., 2009, 19, 2018-2025), 2.00 g of fluorescein, 2.42 g of 3-bromopropene and 4.97 g of anhydrous potassium carbonate were added In the reaction bottle, add 50ml of DMF as a solvent, a trace of iodine as a catalyst, and a trace of hydroquinone as a polymerization inhibitor, and react at 71°C for 25 hours under anhydrous and oxygen-free conditions to obtain a yellow crude product of allyl fluorescein, which is recrystallized from chloroform , the pure product was obtained by column chromatography.
[0025]
[0026] 2. Preparation of carboxypolystyrene copolymerized fluorescent microspheres: Take 0.07g of azobisisobutyronitrile, 0.21g of polyvinylpyrrolidone, 9ml of absolute ethanol and 1.2ml of water respectively, put them in a microreactor, and ultrasonically obtain clarity solution. Add 3.0 g of styrene, 0.11 g of acrylic acid, and 9 mg of allyl fluorescein, pass nitrogen to remove oxygen,...
Embodiment 3
[0028] 1. Synthesis of Allyl Nile Red: According to the literature (J. Mater. Chem., 2009, 19, 2018-2025) method, m-N,N-diethylaminophenol 1.65g, concentrated hydrochloric acid 3.40ml and water 1.70 ml into the reaction flask, stirred in an ice bath until dissolved, controlled the temperature at 0°C, and slowly added an aqueous solution of sodium nitrite (NaNO 2 0.78g, H 2 O 5.9mL), the addition was completed in 30min, reacted at 0-5°C for 4h, filtered to obtain the crude hydrochloride product, and dried in vacuum at 50°C for 8h. Dissolve the crude product in 25ml of boiling ethanol, cool to 40°C, add diethyl ether slowly until crystals appear, place the mixture in the refrigerator for 24 hours, and filter to obtain khaki needle-like crystals of 5-(diethylamino)-2-nitrosophenol .
[0029]
[0030] Put 0.50g of 5-(diethylamino)-2-nitrosophenol, 0.33g of 1,6-dihydroxynaphthalene and 40ml of dry DMF into the reaction flask, stir and heat to reflux, react for 4h and evaporat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| emission peak | aaaaa | aaaaa |
| emission peak | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com