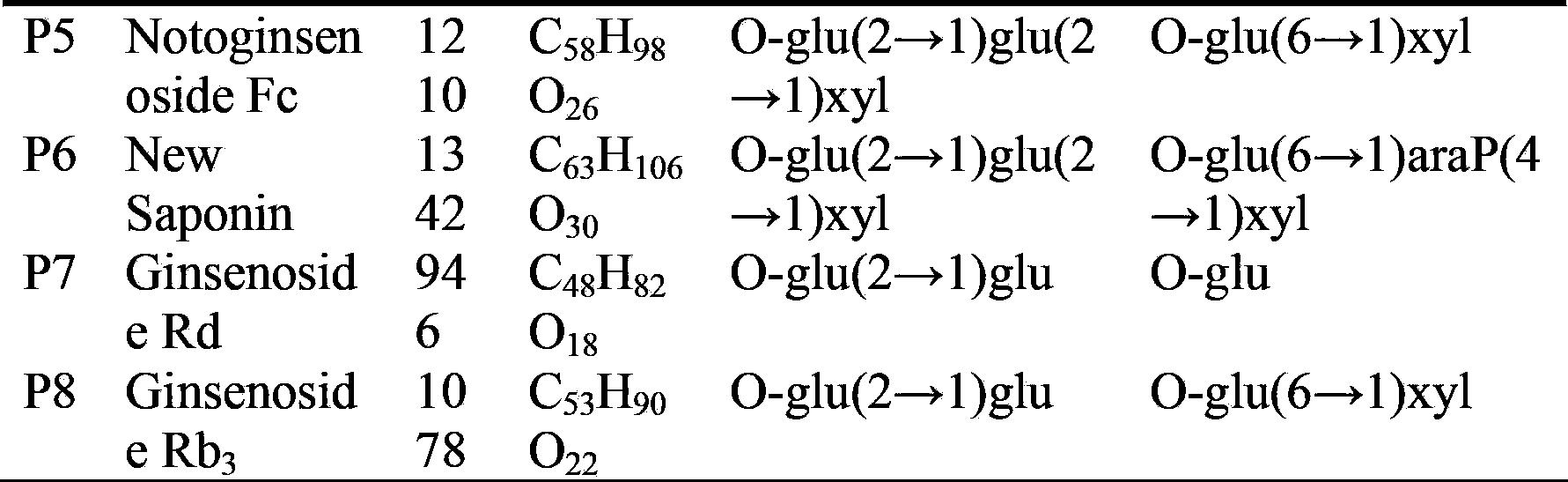Saponin separation and purification method
A technology for separation and purification of saponins, which is applied in the field of separation and purification of saponins, and can solve problems such as rigid chromatographic separation modes, limited types of hydrophilic chromatographic separation materials, and difficulty in discovering new compounds.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1: Preparation of the first dimension fraction of saponin extract
[0022] The water extract of Notoginseng leaf was separated by preparative HPLC (C18 TDE column; water (A) and acetonitrile (B) mobile phase system; elution gradient was 0-5min, volume concentration 20%→32%B; 5-45min, Volume concentration 32%→68%B; 45-50min, volume concentration 68%→100%B; 50-55min, 100%B); during the separation process, an ultraviolet detector was used for detection, and the detection wavelength was 203nm. 1-55min, one collection per minute, a total of 54 components. Fractions 1-54 respectively, saponins mainly concentrated in Fractions 6-30.
Embodiment 2
[0023] Embodiment 2: the preparation of compound P1 and P2
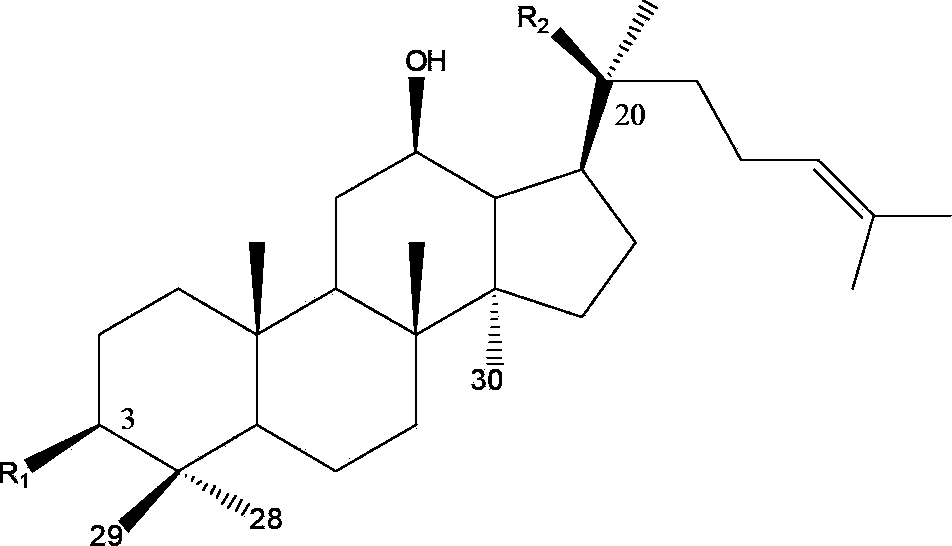
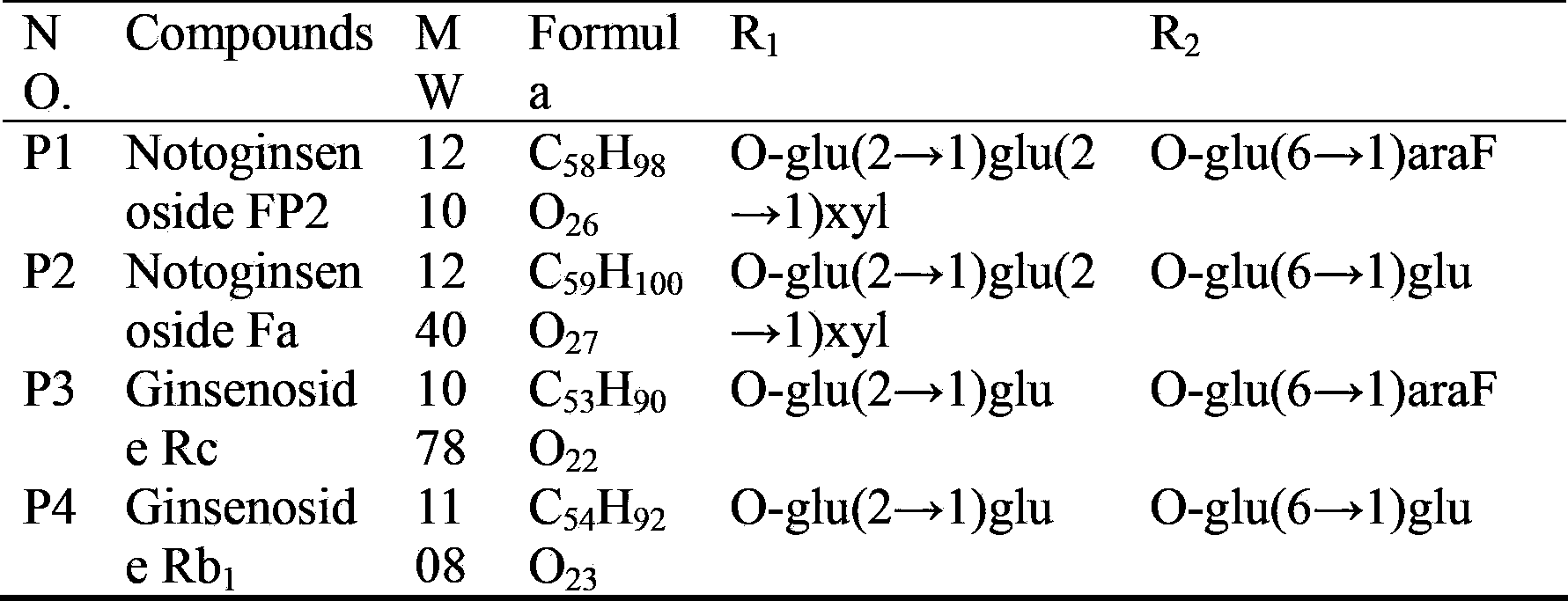
[0024] Fraction 9 was selected for second-dimensional preparative HPLC separation using hydrophilic chromatography mode (the chromatographic column is an amide column; mobile phase system of water (A) and acetonitrile (B); volume concentration 76% B isocratic elution) The flow rate is 20mL / min; the detection wavelength is 203nm; the injection volume is 1mL. Each chromatographic peak was collected, the solvent was recovered separately, and two compounds, P1 and P2, were obtained through NMR experiments. The purity detected by HPLC is greater than 95%, and the data are as follows: P1, white powder, HR-ESI-MS: [M+H] + (m / z):1211.6409. 1 H NMR(600MHz,pyridine-d5)δ:0.77(1H,s,H-19),0.92(2H,s,H-18,30),1.08(1H,s,H-29),1.25(1H, s,H-28),1.60(1H,s,H-21),1.62(1H,s,H-27),1.64(1H,s,H-26),3.28(1H,dd,J=4.2, 11.4,H-3),5.29(1H,t,J=7.2,H-24),4.92(1H,d,J=7.8),5.50(1H,d,J=7.8),5.40(1H,d, J=6.6),5.13(1H,d,J=7.8),4.85(1H,brs). 13 C ...
Embodiment 3
[0025] Embodiment 3: the preparation of compound P3-P6
[0026] Fraction 11 was selected for second-dimensional preparative HPLC separation using hydrophilic chromatography mode (the chromatographic column is an amide column; mobile phase system of water (A) and acetonitrile (B); volume concentration 80% B isocratic elution) The flow rate is 20mL / min; the detection wavelength is 203nm; the injection volume is 1mL. Each chromatographic peak was collected, the solvent was recovered separately, and four compounds P3-P6 were obtained through NMR experiments. The purity detected by HPLC is greater than 95%, and the data are as follows: P3, white powder, HR-ESI-MS: [M+H] + (m / z):1079.5989. 1 H NMR(600MHz,pyridine-d5)δ:0.78(1H,s,H-19),0.92(1H,s,H-30),0.93(1H,s,H-18),1.08(1H,s, H-29),1.26(1H,s,H-28),1.60(1H,s,H-21),1.62(1H,s,H-27),1.64(1H,s,H-26),3.24 (1H,dd,J=4.2,11.4,H-3),5.29(1H,t,J=6.6,H-24),4.90(1H,d,J=7.8),5.35(1H,d,J= 7.8),5.12(1H,d,J=7.8),4.85(1H,brs). 13 C NMR: see Tab...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com