Novel C3N4/ZnO/Fe2O3 composite photocatalyst and preparation method as well as application thereof
A composite catalyst, C3N4 technology, applied in the chemical field, can solve the problems of operator casualties, environmental pollution, low degradation rate of methylene blue, etc., and achieve the effect of improving the utilization rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
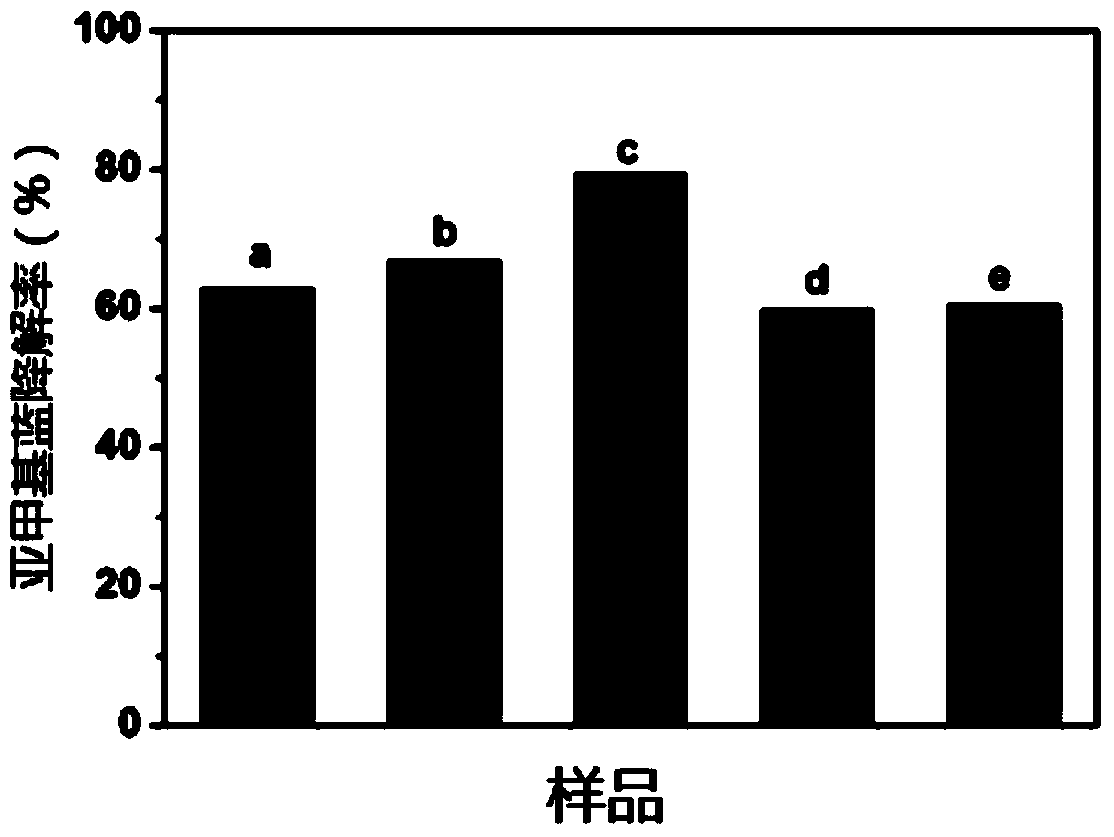
Embodiment 1
[0075] (1) Accurately weigh 3.0g of dicyandiamide and 0.2928g of barbituric acid, put them in a 200mL beaker, add 15mL of distilled water and stir evenly, place the beaker in a 90°C CHH-4 constant temperature water bath, and keep stirring until Evaporate the water in the beaker to dryness; take out the beaker and place it in a blast drying oven at 100°C until the mixture is dry; grind the mixture into a crucible and place it in a muffle furnace, calcinate at 550°C for 5.0h, and wait to cool to room temperature After grinding finely, C 3 N 4 catalyst;
[0076] (2) Accurately weigh 1.629g of ferric nitrate nonahydrate and 0.220g of zinc nitrate hexahydrate, put the above-mentioned three reagents in a 20mL beaker, add 10mL of deionized water, add a stirring magnet after the dissolution is complete, and put The mixed solution was stirred on a magnetic stirrer for 30 minutes, and the evenly stirred beaker was placed in a 90°C constant temperature water bath until the solvent was ...
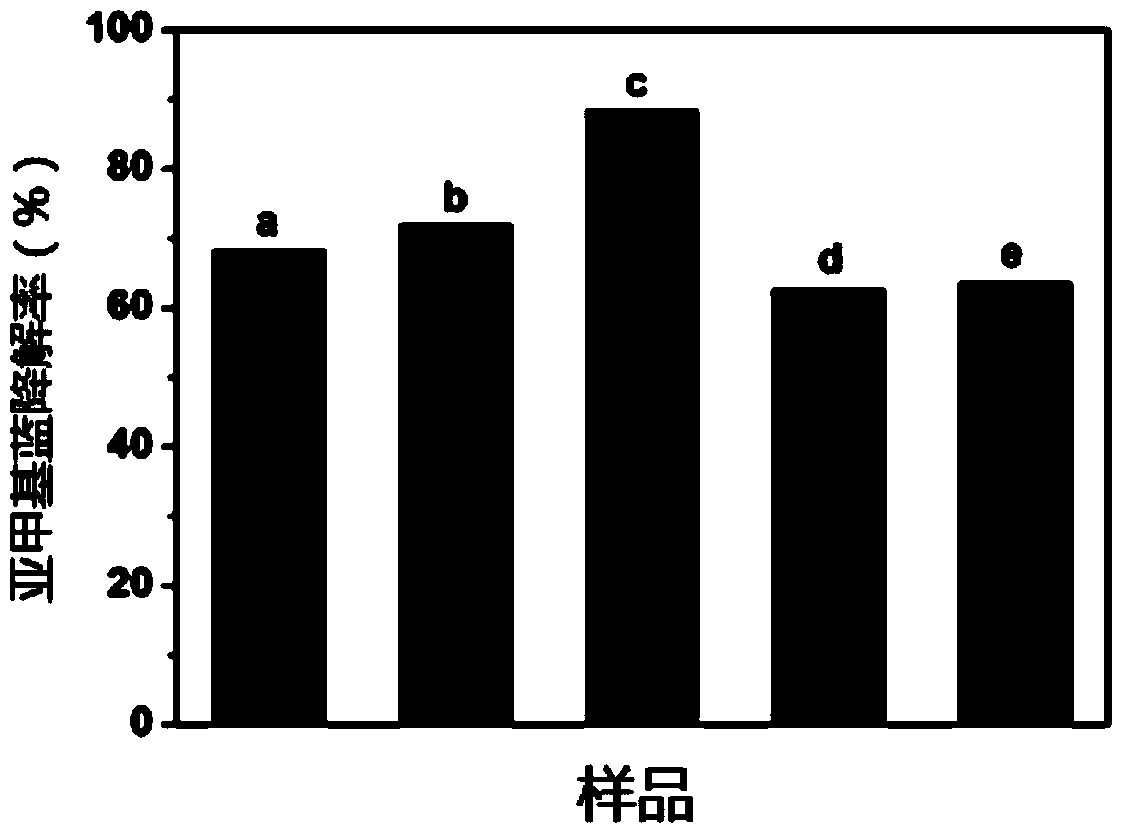
Embodiment 2
[0079] (1) Accurately weigh 5.0g of melamine and 1.0g of barbituric acid, put them in a beaker, add 10mL of distilled water and stir evenly, place the beaker in a constant temperature water bath at 80°C, and keep stirring until the water in the beaker evaporates to dryness; take out Place the beaker in a blast drying oven at 80°C until the mixture is dry; grind the mixture into a crucible and place it in a muffle furnace, calcinate at 530°C for 5.0h, cool to room temperature, and grind to obtain C 3 N 4 catalyst;
[0080] (2) Accurately weigh 3.257g of anhydrous ferric nitrate and 0.440g of anhydrous zinc nitrate, put the above three reagents in a beaker, add 15mL of deionized water, add a stirring magnet after the dissolution is complete, and mix The solution was stirred on a magnetic stirrer for 30 minutes, and the evenly stirred beaker was placed in a 90°C constant temperature water bath until the solvent was evaporated to dryness, and the mixture after the solvent was eva...
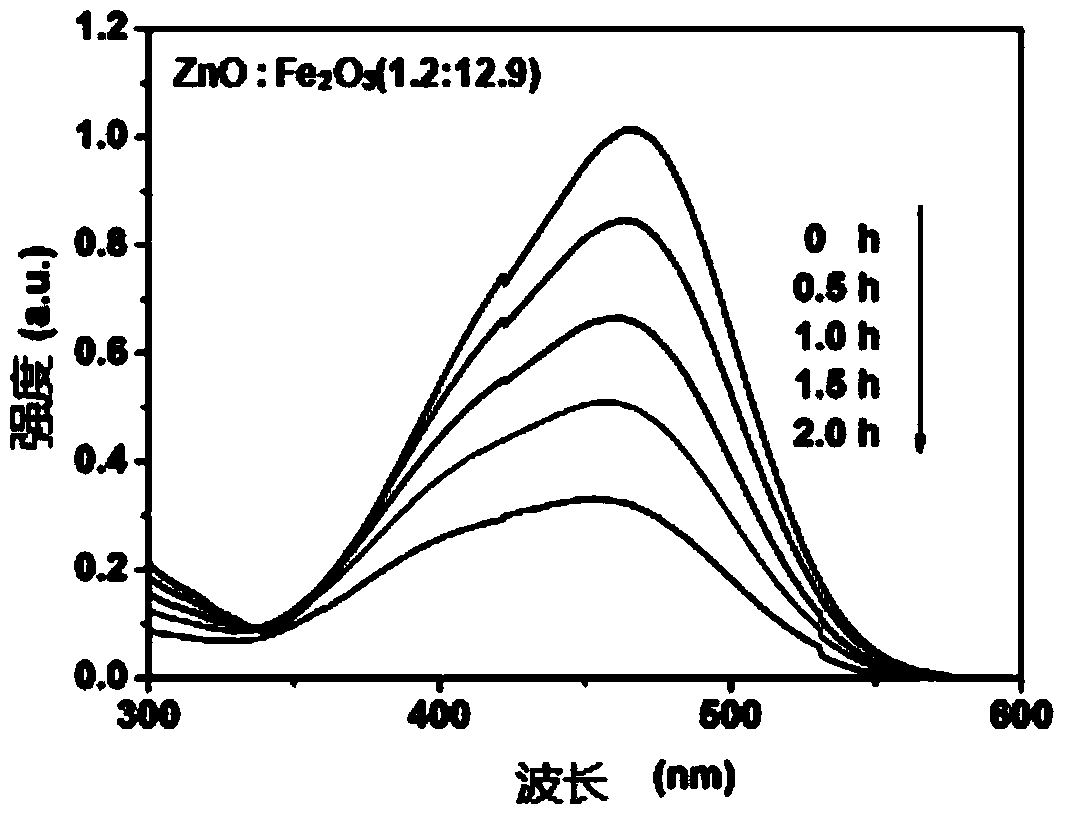
Embodiment 3
[0083] (1) Accurately weigh 15.0g of dicyandiamide and 1.0g of barbituric acid, put them in a beaker, add 30mL of distilled water and stir evenly, place the beaker in a constant temperature water bath at 100°C, and keep stirring until the water in the beaker evaporates Dry; take out the beaker and place it in a blast drying oven at 110°C until the mixture is dry; grind the mixture into a crucible and place it in a muffle furnace, calcinate at 570°C for 7.0h, and grind it after cooling to room temperature. Made C 3 N 4 catalyst;
[0084] (2) Accurately weigh 2.600g of ferric nitrate nonahydrate and 0.09167g of zinc nitrate hexahydrate, put the above three reagents in a beaker, add 5mL of deionized water, add a stirring magnet after the dissolution is complete, and mix The solution was stirred on a magnetic stirrer for 30 minutes, and the evenly stirred beaker was placed in a constant temperature water bath at 80°C until the solvent was evaporated to dryness, and the mixture a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com