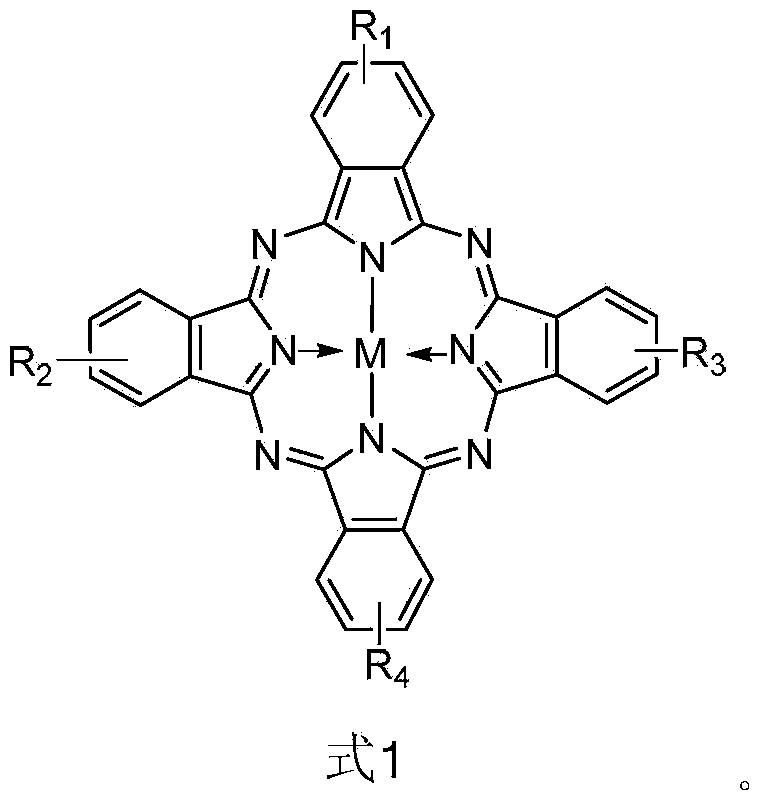
Mesoporous-carbon loaded metal phthalocyanine with catalytic activity and preparation methods
A technology for supporting metals and metal phthalocyanines, applied in the direction of catalyst carriers, chemical instruments and methods, organic compounds/hydrides/coordination complex catalysts, etc., to achieve the effects of preventing aggregation, high catalytic activity, and good catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Weigh 0.05g of tetraaminocobalt phthalocyanine and fully dissolve it in 40mL of dimethyl sulfoxide, add it dropwise to 100mL of dimethyl sulfoxide solution containing 1g of mesoporous carbon vigorously stirred at 85°C, and continue to stir evenly for 30min. Add 1mL of isoamyl nitrite (97%), and react at 85°C for 12h. The reacted solution was poured out, centrifuged to obtain a crude product, then repeatedly washed with N,N-dimethylformamide, water, and ethanol, and dried at 80°C to obtain 0.97 g of mesoporous carbon-supported cobalt phthalocyanine.
Embodiment 2
[0027] Weigh 0.5g of tetraaminozinc phthalocyanine fully dissolved in 100mL N, N-dimethylformamide, and add dropwise to 300mL N, N-dimethylformamide solution containing 5g of mesoporous carbon under vigorous stirring at 75°C, After continuing to stir evenly for 30 minutes, add 5 mL of isoamyl nitrite (97%) and react at 85°C for 18 hours. The reacted solution was poured out and centrifuged to obtain a crude product, which was then repeatedly washed with N,N-dimethylformamide, water and ethanol, and dried at 95°C to obtain 5.11 g of mesoporous carbon-supported zinc phthalocyanine.
Embodiment 3
[0029] Weigh 0.1g of monoaminotrinitrocopper phthalocyanine and fully dissolve it in 60mL of dimethyl sulfoxide, add it dropwise to 120mL of dimethyl sulfoxide solution containing 1.5g of mesoporous carbon which is vigorously stirred at 80°C, and continue to stir After homogenizing for 30 minutes, add 2 mL of isoamyl nitrite (97%) and react at 85°C for 24 hours. The reacted solution was poured out and centrifuged to obtain a crude product, which was then repeatedly washed with N,N-dimethylformamide, water, and ethanol, and dried at 80°C to obtain 1.43 g of mesoporous carbon-supported trinitrocobalt phthalocyanine.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com