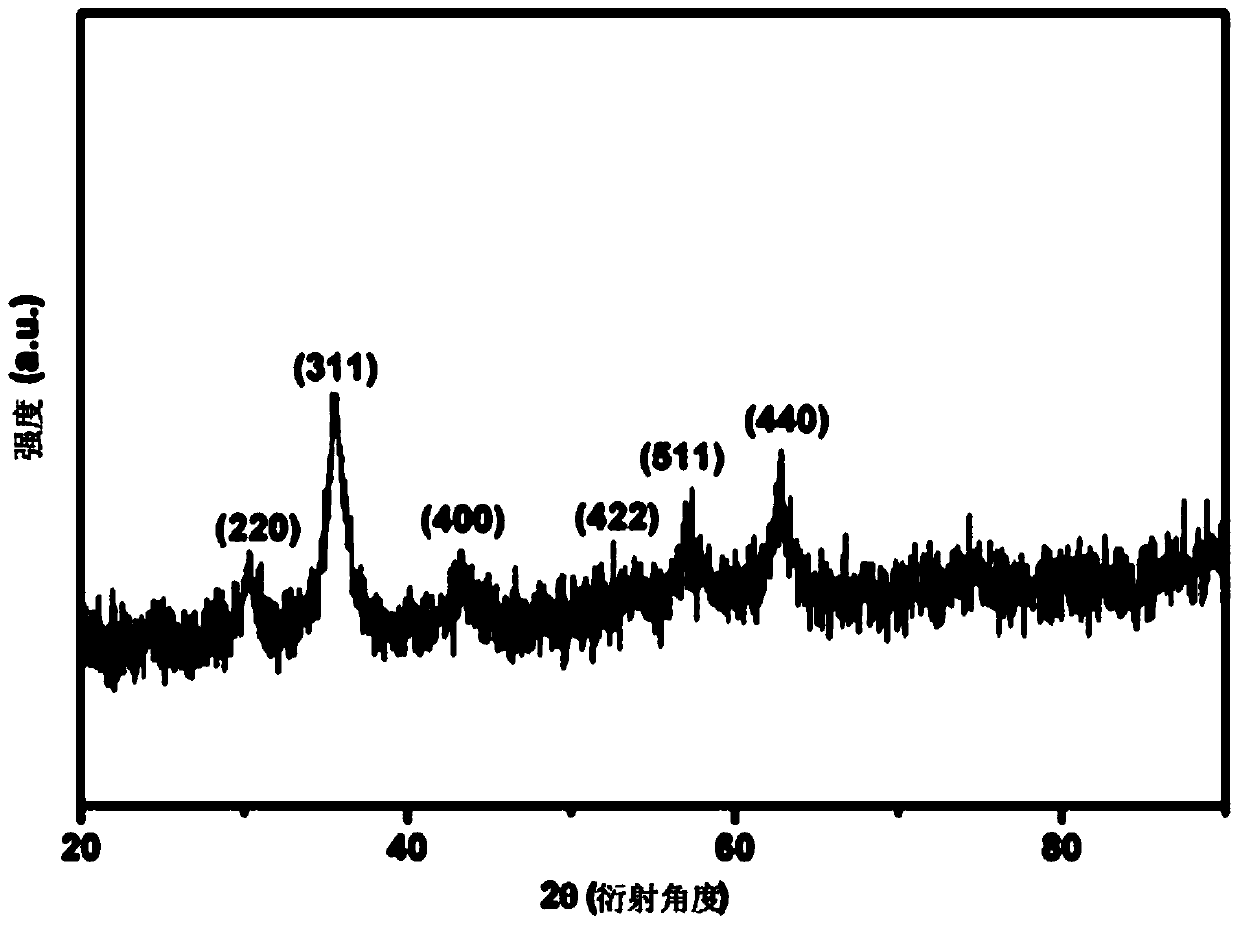
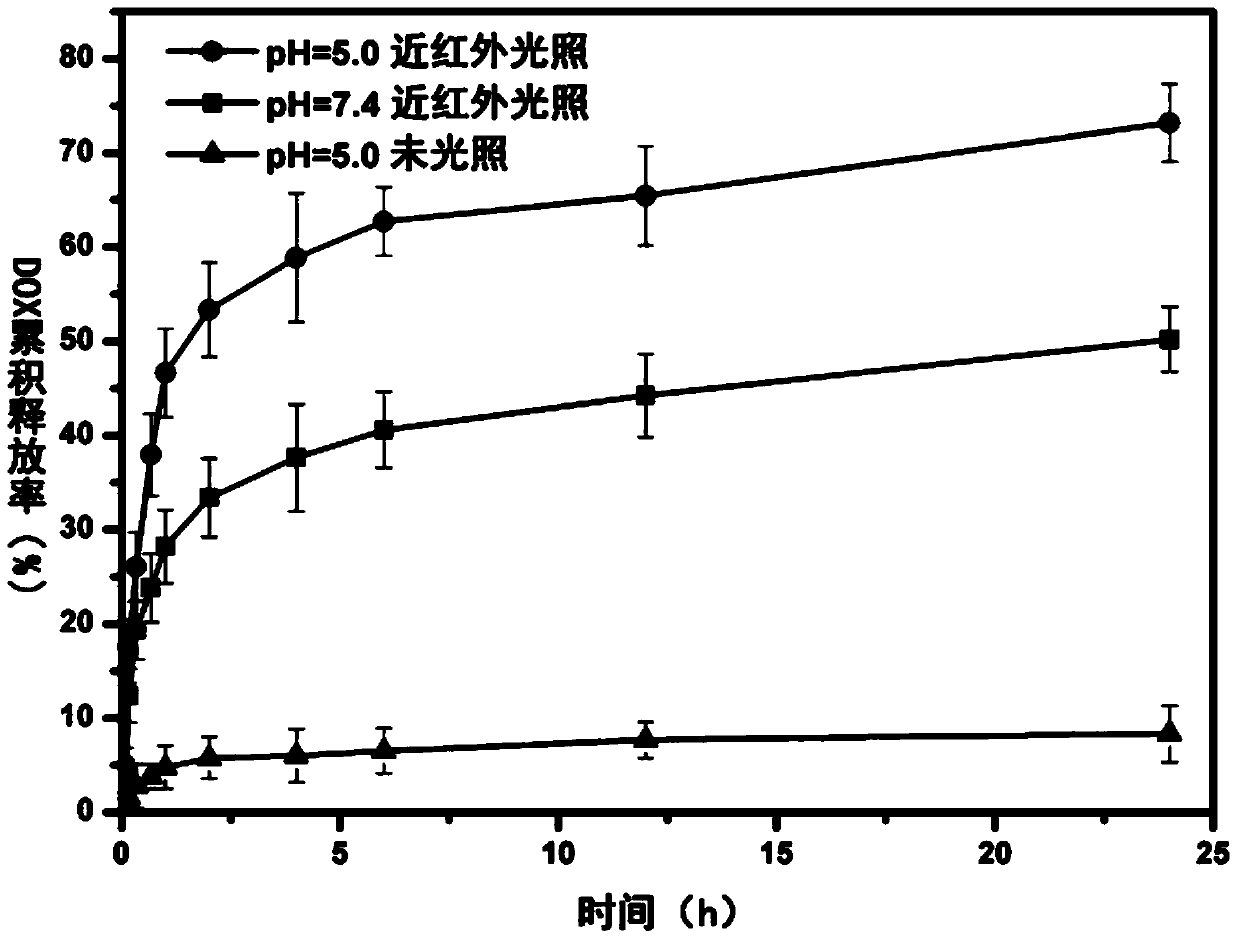
Amphiphilic polymer and preparation thereof as well as magnetic hollow nano-drug carrier and preparation method thereof
An amphiphilic polymer, polymer technology, used in drug combinations, pharmaceutical formulations, anti-tumor drugs, etc., can solve problems such as limiting, reducing light intensity, cell and tissue damage, and achieving strong tissue penetration, reducing Injury, increase the effect of drug load
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Synthesis of hollow porous Fe3O4 (HPFe 3 o 4 ),Specific steps are as follows:
[0055] Add 0.15mL oleylamine to 20mL 1-octadecene, degas with argon at 120°C for 2h, then raise the temperature to 180°C, immediately add 1.4mL iron pentacarbonyl (protected by argon), stir continuously for 30min, then cool to room temperature, pour off the supernatant, add a mixture of n-hexane and oleylamine to disperse, add isopropanol to precipitate, centrifuge, wash twice with a mixture of n-hexane and oleylamine, and finally obtain nanoparticles dispersed in In a mixture of 15mL n-hexane and 0.01mL oleylamine. The obtained nanoparticles are Fe / Fe 3 o 4 Core-shell nanospheres.
[0056] Add 30 mg of trimethylamine N-oxide to 20 mL of 1-octadecene, degas with argon at 130 ° C for 2 h, add 80 mg of the above-mentioned Fe / Fe 3 o 4 Keep the n-hexane solution at 130°C and continue to stir to remove the n-hexane, then keep it at 130°C for 12h, raise the temperature to 250°C, keep it for...
Embodiment 2
[0058] Embodiment 2 and embodiment 3 are the synthesis of hydrophobic monomer
[0059] Example 2
[0060] Synthesis of 7-didodecylamino-4-hydroxymethylcoumarin methacrylate (DDACMM):
[0061] Add 22g o-aminophenol to 150mL ethyl acetate containing 25g potassium bicarbonate and 10mL water, add 18mL methyl chloroformate dropwise under ice bath with constant stirring, react for 1h, add 50mL water and stir for 3h, separate the organic phase After washing with water, 1 mol / L sulfuric acid solution, water and saturated brine, drying over magnesium sulfate, rotary evaporation and recrystallization with benzene, compound 1 was obtained.
[0062] 23g of compound 1 was added to a mixture of 25mL ethyl acetoacetate and 60mL of concentrated sulfuric acid, stirred for 2 hours, then added 250mL of ice water, continued stirring until the crystallization stopped, filtered, the filter cake was washed with water, methanol, ether and dried to obtain compound 2.
[0063] Add 28g of compound 2 i...
Embodiment 3
[0068] Synthesis of 7-Dihexadecylamino-4-Hydroxymethylcoumarin Methacrylate (DHACMM)
[0069] Compound 3 was synthesized with reference to Example 2.
[0070]Dissolve 2.66g of compound 3 and 47.12g of hexadecyl bromide in 250mL of N,N-dimethylformamide, add 7.4g of cesium carbonate, reflux for 12h, distill DMF under reduced pressure, add water and ethyl acetate to separate the liquid, The organic phase was washed with water and saturated brine, dried over anhydrous magnesium sulfate, and passed through the column with ethyl acetate:petroleum ether=1:4 after rotary evaporation to obtain compound 6. Compound 6 H NMR spectrum results are as follows: 1 H NMR (CDCl 3 ,400MHz):δ7.36(d,J=8.0Hz,1H,Ar-H),6.55(d,J=8.0Hz,1H,Ar-H),6.49(s,1H,Ar-H),5.99 (s,1H,Ar-H),3.64(t,J=12.0Hz,1H,N-CH 2 CH 2 ),3.14(t,J=12.0HZ,3H,N-CH 2 CH 2 ),2.34(s,3H,CH 3 ),1.72-1.47(m,4H,N-CH 2 ),1.45-1.05(m,44H,CH 2 ),0.88(t,J=16Hz,6H,CH 3 ).
[0071] 9.86g compound 6 was dissolved in 150mL xylene, 13.4g...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com