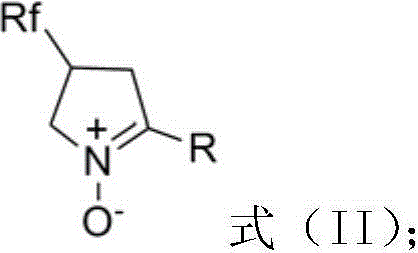
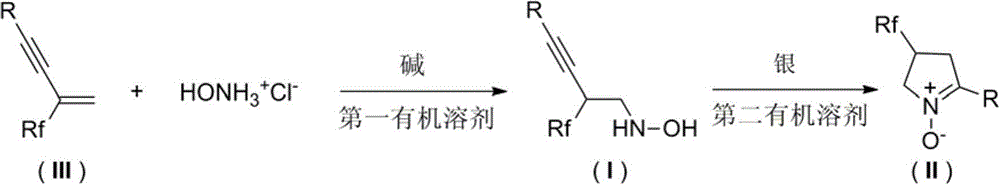
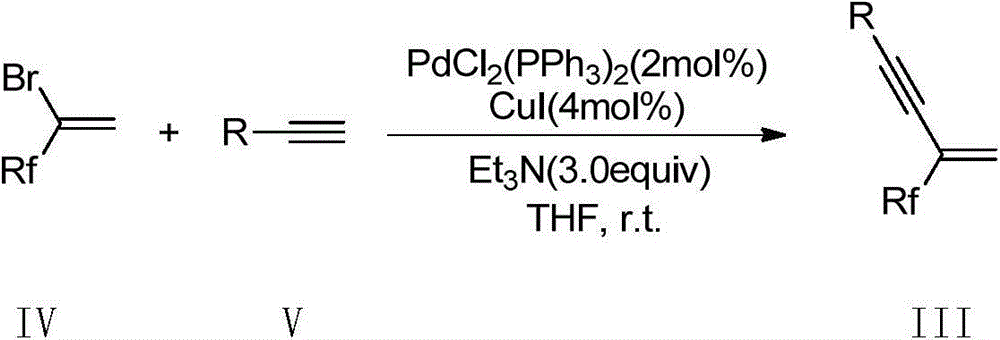Multi-halothane five-member cyclic nitrone derivative and preparation method thereof
A technology of polyfluoroalkyl and derivatives, applied in oxime preparation, organic chemistry, etc., can solve problems such as difficult separation, long reaction time, synthesis of polyfluoroalkyl-containing nitrone compounds that have not been reported in literature, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The raw materials 3-trifluoromethyl-3-buten-1-ynylbenzene (0.2 mmol), hydroxylamine hydrochloride (0.3 mmol), and dichloromethane (2.0 ml) were placed in a reaction flask. Triethylamine (0.34 mmol) was added at 0° C. and stirred for 24 h, and the reaction was checked by TLC until the raw material disappeared completely. The solvent was distilled off under reduced pressure and the crude product was directly purified by flash column chromatography on silica gel (petroleum ether: ethyl acetate = 5: 1) to obtain pure product I-1 (32.1mg, 70 %).
[0042]
[0043] 1 H NMR (400MHz, CDCl 3 )δ7.47(dd, J=7.7, 1.6Hz, 2H), 7.37-7.29(m, 3H), 5.82(s, 1H), 5.62(brs, 1H), 4.03-3.90(m, 1H), 3.45 (dd, J=13.2, 4.4Hz, 1H), 3.17(dd, J=13.2, 9.6Hz, 1H). 19 F NMR (377MHz, CDCl 3 )δ-69.61. 13 C NMR (100MHz, CDCl 3 )δ131.97, 128.90, 128.33, 124.83 (q, J=278.0Hz), 121.73, 85.90, 80.13, 52.21, 36.20 (q, J=30.0Hz).MS (70eV): m / z (%): 229 (M + , 1.17), 46(100). HRMS calculation: C 11 h...
Embodiment 2
[0045] Starting material 1-methyl-4-(3-trifluoromethyl)-3-buten-1-ynylbenzene (0.2mmol), hydroxylamine hydrochloride (0.4mmol), 1,2-dichloroethane (2.0 ml) placed in the reaction flask. Tetramethylethylenediamine (0.44 mmol) was added at room temperature and stirred for 24 h. The reaction was detected by TLC until the raw material disappeared completely. The solvent was distilled off under reduced pressure and the crude product was directly purified by flash column chromatography on silica gel (petroleum ether: ethyl acetate = 10:1) to obtain pure product I-2 (32.1mg, 66 %).
[0046]
[0047] 1 H NMR (400MHz, CDCl 3 )δ7.36(d, J=8.1Hz, 2H), 7.12(d, J=8.1Hz, 2H), 6.03(brs, 1H), 5.62(brs, 1H), 4.02-3.88(m, 1H), 3.44(dd, J=13.2, 4.4Hz, 1H), 3.16(dd, J=13.2, 9.6Hz, 1H), 2.35(s, 3H). 19 F NMR (377MHz, CDCl 3)δ-69.67. 13 C NMR (100MHz, CDCl 3 )δ139.10, 131.85, 129.06, 124.86 (q, J=278.0Hz), 118.64, 86.08, 79.36, 52.22, 36.19 (q, J=30.0Hz), 21.49.MS (70eV): m / z (%) : 243(M...
Embodiment 3
[0049] The raw material 1-methoxy-4-(3-trifluoromethyl)-3-butene-1-ynylbenzene (0.2mmol), hydroxylamine hydrochloride (0.36mmol), and chloroform (1.6ml) were placed in in the reaction vial. Triethylenediamine (0.4 mmol) was added at 5° C. and stirred for 30 h, and the reaction was detected by TLC until the raw material disappeared completely. The solvent was distilled off under reduced pressure and the crude product was directly purified by flash column chromatography on silica gel (petroleum ether: ethyl acetate = 1: 1) to obtain pure product I-3 (38.9mg, 75%).
[0050]
[0051] 1 H NMR (400MHz, CDCl 3 )δ7.40(d, J=8.8Hz, 2H), 6.83(d, J=8.8Hz, 2H), 5.99(brs, 2H), 4.02-3.87(m, 1H), 3.81(s, 3H), 3.43(dd, J=13.2, 4.4Hz, 1H), 3.15(dd, J=13.2, 9.6Hz, 1H). 19 F NMR (377MHz, CDCl 3 )δ-69.72. 13 C NMR (100MHz, CDCl 3 )δ160.00, 133.44, 124.83(q, J=278.0Hz), 113.92, 113.76, 85.92, 78.65, 78.61, 77.32, 77.00, 76.68, 55.25, 52.16, 36.12(q, J=30.0Hz).MS(70eV ): m / z (%): 259 (M ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com