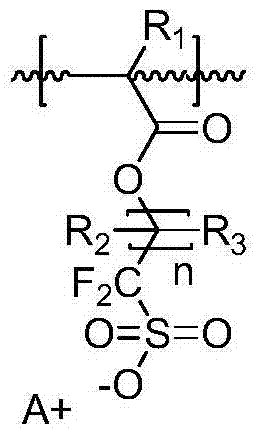
Novel onium salt compound, acid amplifier derived therefrom, and resist composition comprising same
An acid enhancer and compound technology, which can be applied in the fields of organic chemistry, sulfonate preparation, photoengraving process of pattern surface, etc., and can solve problems such as few photons
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
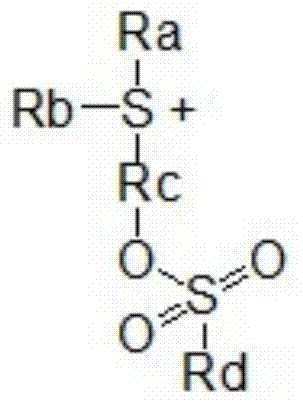
[0133] Specifically, the compound of the chemical formula 3 can be prepared by the following preparation method, which includes the following steps: making the hydroxyl ring compound of the following chemical formula 5 and the sulfonyl halide of the following chemical formula 6 in the presence of a basic compound A step of reacting to obtain a compound of the following chemical formula 7; and a step of reacting the compound of the chemical formula 7 with a sulfoxide compound of the following chemical formula 8 and trifluoromethanesulfonic anhydride.
[0134] chemical formula 5
[0135]
[0136] chemical formula 6
[0137]
[0138] chemical formula 7
[0139]
[0140] chemical formula 8
[0141]
[0142] In said chemical formula,
[0143] R a , R b and R d same as defined above,
[0144] X is selected from the group consisting of an aryl group with 6 to 30 carbon atoms, a heteroaryl group with 5 to 30 carbon atoms, and a heterocycloalkyl group with 2 to 30 ca...
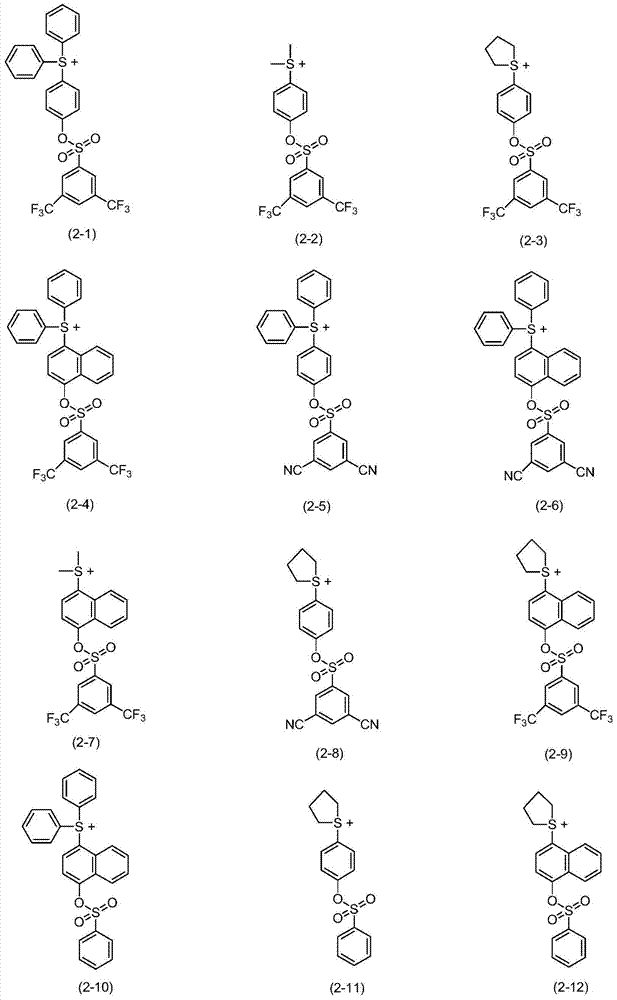
Synthetic example 1
[0256] In a round bottom flask, dissolve 6.9g of phenol and 23g of 3,5-bis-trifluoromethyl-benzenesulfonyl chloride in 200ml of dichloromethane Then, to the solution obtained by stirring, 8.9 g of triethylamine (triethylamine) was slowly added dropwise at normal temperature, and stirred for 2 hours. After confirming the completion of the reaction by TLC, the resulting reaction solution was washed with an aqueous sodium hydroxide solution and then twice with distilled water, and the organic layer was separated and collected. Obtained 3,5-bis-trifluoromethyl-benzenesulfonic acid phenyl ester (3,5-Bis-trifluoromethyl-benzenesulfonic acid phenyl ester) (i) after removing the solvent component in the collected organic layer with a vacuum distiller (22.2 g, 81% yield). The structure of the obtained compound was confirmed by 1H NMR.
[0257] 1 H NMR (CDCl 3 , internal standard: tetramethylsilane): (ppm) 7.00 (d, 2H), 7.3 ~ 7.4 (t, 3H), 8.2 (s, 1H), 8.3 (s, 1H)
[0258] Reaction 1 ...
Synthetic example 2
[0261] After putting 5 g of 3,5-bis-trifluoromethyl-benzenesulfonic acid phenyl ester (i) and 3 g of phenyl sulfone (phenyl sulfoxide) synthesized in the synthesis example 1 into a round bottom flask, as a reaction solvent Dissolution was performed using 50 ml of dichloromethane. After preparing an ice bath (bath) and cooling the reaction flask to -10°C, 4.8 g of trifluoromethanesulfonic anhydride (trifluoromethanesulfonic anhydride) was slowly dropped into the reactor. After completion of the dropwise addition, stirring was carried out for 2 hours while maintaining the internal temperature of the reactor at -10°C. After confirming the completion of the reaction with TLC, the reaction solution obtained as a result was added to potassium carbonate (K 2 CO 3 ) in the aqueous solution for vigorous stirring to neutralize. After the neutralization process was completed, it was washed twice with distilled water, and only the organic layer was separated and collected. The solvent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com