Method for detecting 3-hydroxycotinine in urea
A technology of hydroxycotinine and urine, which is applied in the field of physical and chemical testing of tobacco alkaloids, achieves the effects of low detection limit, small impact, and easy popularization and application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] 1. Preparation of chemical reagents: (3R,5S)-3-hydroxycotinine (CAS No. 34834-67-8), (3S,5S)-3-hydroxycotinine (CAS No. 37096-14-3 ),(3R,5S)-3-hydroxycotinine-d 3 (CAS No. 159956-78-2), beta-glucuronidase (type IX-A, from Escherichia coli).
[0025] 2. Standard curve establishment:
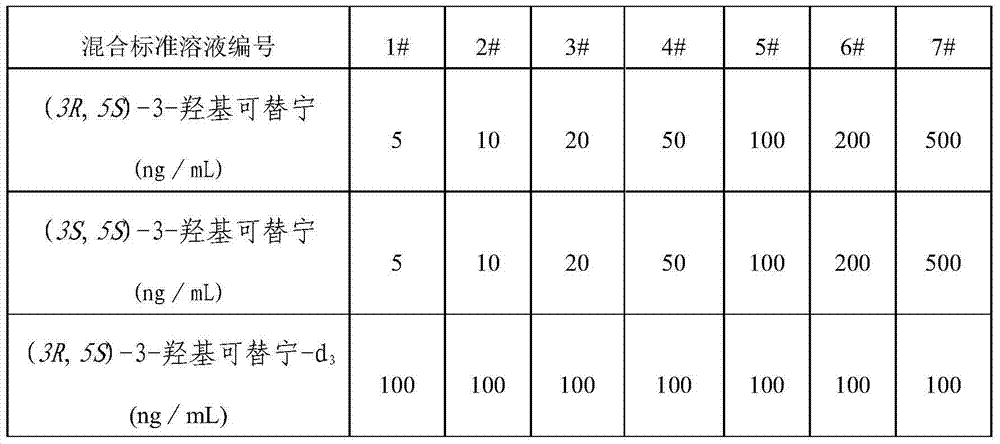
[0026] Table 2 Proportion of mixed standard solution
[0027]
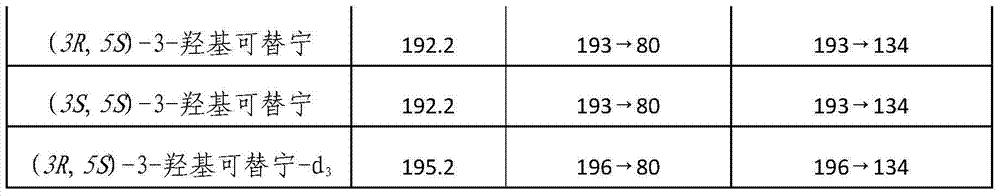
[0028] Using acetonitrile as solvent, prepare (3R,5S)-3-hydroxycotinine, (3S,5S)-3-hydroxycotinine and (3R,5S)-3-hydroxycotinine-d according to Table 2 3 The mixed standard solution, determine the chromatographic peaks of (3R, 5S)-3-hydroxyl cotinine and (3S, 5S)-3-hydroxyl cotinine by retention time; In the HPLC-MS-MS chromatogram (3R, The concentration of 5S)-3-hydroxycotinine and (3R,5S)-3-hydroxycotinine-d 3 The ratio of the concentration is the abscissa, with the quantitative ion-pair peak area of (3R, 5S)-3-hydroxyl cotinine in the chromatogram and (3R, 5S)-3-hydroxyl cotinine-d 3 The ratio of the quantitative i...
Embodiment 2
[0033] Repeat Example 1 with the following differences: Shake the urine sample after thawing at room temperature, accurately pipette 1.0 mL into a 10 mL test tube, and add 20 μL of (3R,5S)-3-hydroxyl at a concentration of 10 mg / mL Cotinine-d 3 solution, then add 0.2mL of sodium phosphate buffer solution (pH6.0, 0.1mol / L) and 100μL of β-glucuronidase (1000U / mL urine), mix thoroughly and place in a microwave-assisted extraction apparatus at 37°C Enzymatic hydrolysis at constant temperature in the dark for 2 minutes. Add 10 mL of ultrapure water to the urine sample after enzymatic hydrolysis, filter it with a 0.2 μm aqueous phase filter membrane, and perform HPLC-MS-MS detection. Use (3R,5S)-3-hydroxycotinine and (3S,5S)-3-hydroxycotinine standard solution working curves to calculate the (3R,5S)-3-hydroxycotinine in the sample to be tested. Tinine and (3S,5S)-3-hydroxycotinine, and then converted to the concentration of (3R,5S)-3-hydroxycotinine in the urine is 3919ng / mL, (3S,5...
Embodiment 3
[0035] Repeat Example 1 with the following differences: Shake the urine sample after thawing at room temperature, accurately pipette 0.5 mL into a 10 mL test tube, add 10 μL of (3R,5S)-3-hydroxyl at a concentration of 10 mg / mL Cotinine-d 3 solution, then add 0.2mL of sodium phosphate buffer solution (pH6.0, 0.1mol / L) and 100μL of β-glucuronidase (1000U / mL urine), mix thoroughly and place in a constant temperature shaker at 37 Enzyme hydrolysis overnight at constant temperature in the dark. Add 10 mL of ultrapure water to the urine sample after enzymatic hydrolysis, filter it with a 0.2 μm aqueous phase filter membrane, and perform HPLC-MS-MS detection. Use (3R,5S)-3-hydroxycotinine and (3S,5S)-3-hydroxycotinine standard solution working curves to calculate the (3R,5S)-3-hydroxycotinine in the sample to be tested. Tinine and (3S,5S)-3-hydroxycotinine, and then converted to the concentration of (3R,5S)-3-hydroxycotinine in the urine is 1793.8ng / mL, (3S,5S)-3 The concentration...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com