Nonaqueous electrolyte containing sulfonyl fluoride imidogen lithium salt as well as application of electrolyte
A non-aqueous electrolyte, fluorosulfonyl technology, applied in circuits, electrical components, battery electrodes, etc., can solve the problem of not changing and completely eliminating the root cause.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
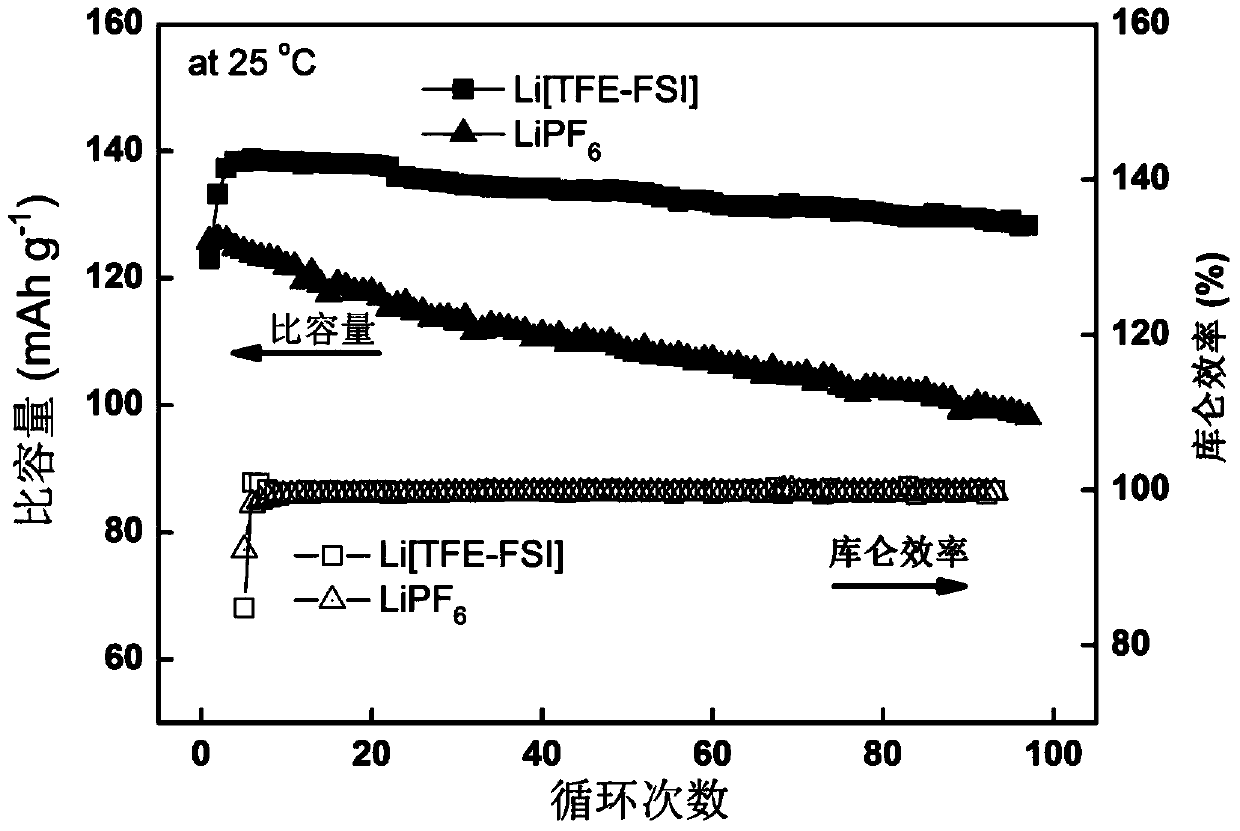
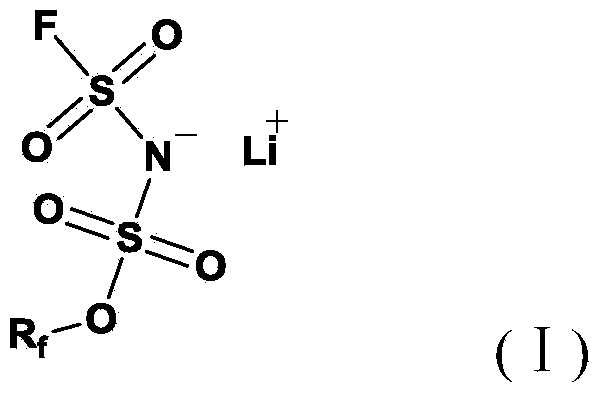
[0039] Examples 1-16 relate to Li[(FSO 2 )(CF 3 CH 2 OSO 2 )N](Li[TFE-FSI]) conductive lithium salt non-aqueous electrolyte in battery applications.
[0040] Example 1
[0041] Li[TFE-FSI]–EC / EMC(3:7,v / v) Electrolyte Cycle Evaluation at Room Temperature
[0042] 1) Fabrication of positive electrode: LiCoO 2 Powder, carbon black (particle size 1000nm), polyvinylidene fluoride (PVDF) and N,N-dimethylpyrrolidone (NMP) are mixed to make a uniform slurry, and the slurry is evenly coated on an aluminum foil (15 μm) current collector on, then dried and rolled to obtain LiCoO 2 Cathode material. Bake at 120°C for 12 hours, in the dried pole piece, LiCoO 2 It accounts for 94% of the total coating, binder accounts for 4%, and carbon black accounts for 2%. Then the obtained pole piece was cut into a disc with a diameter of 12 mm as the positive pole.
[0043] 2) Preparation of negative electrode: Mix artificial graphite, polyvinylidene fluoride (PVDF) and N,N-dimethylpyrrolidon...
Embodiment 2
[0048] LiPF 6 – EC / EMC (3:7, v / v) electrolyte cycle evaluation at room temperature
Embodiment 3
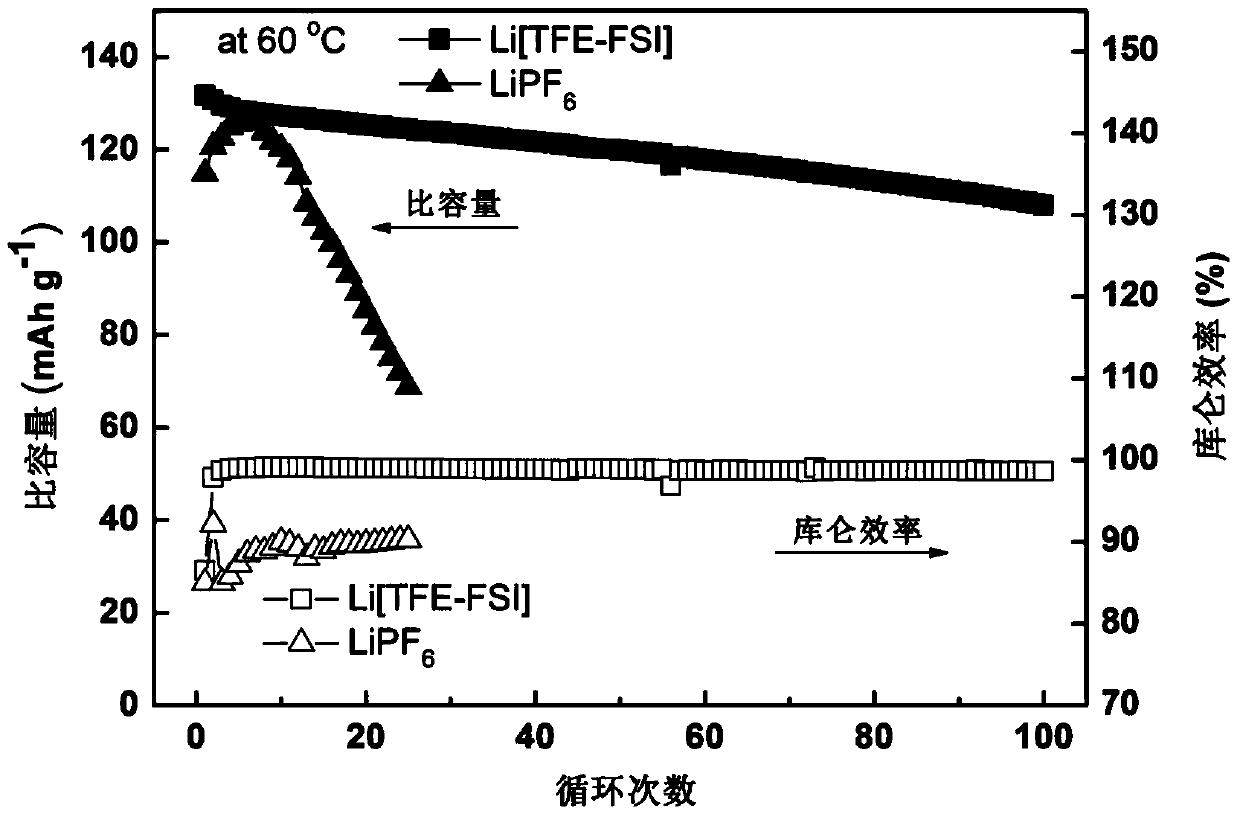
[0053] Evaluation of Li[TFE-FSI]–EC / EMC(3:7,v / v) Electrolyte for High Temperature Cycle
[0054] The same non-aqueous electrolyte as in Example 1 was used to assemble the same battery as in Example 1, and a high-temperature cycle performance test was performed. Test conditions: The assembled battery is directly subjected to a high-temperature cycle test in a constant temperature test box at 60°C, and the cut-off voltage is 4.2–2.75V. The charge rate is 0.5C, and the discharge rate is 0.2C. The test data of this embodiment is shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com