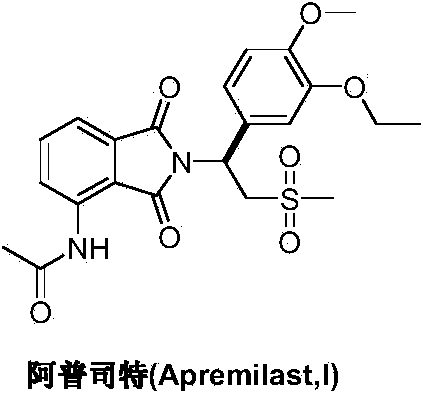
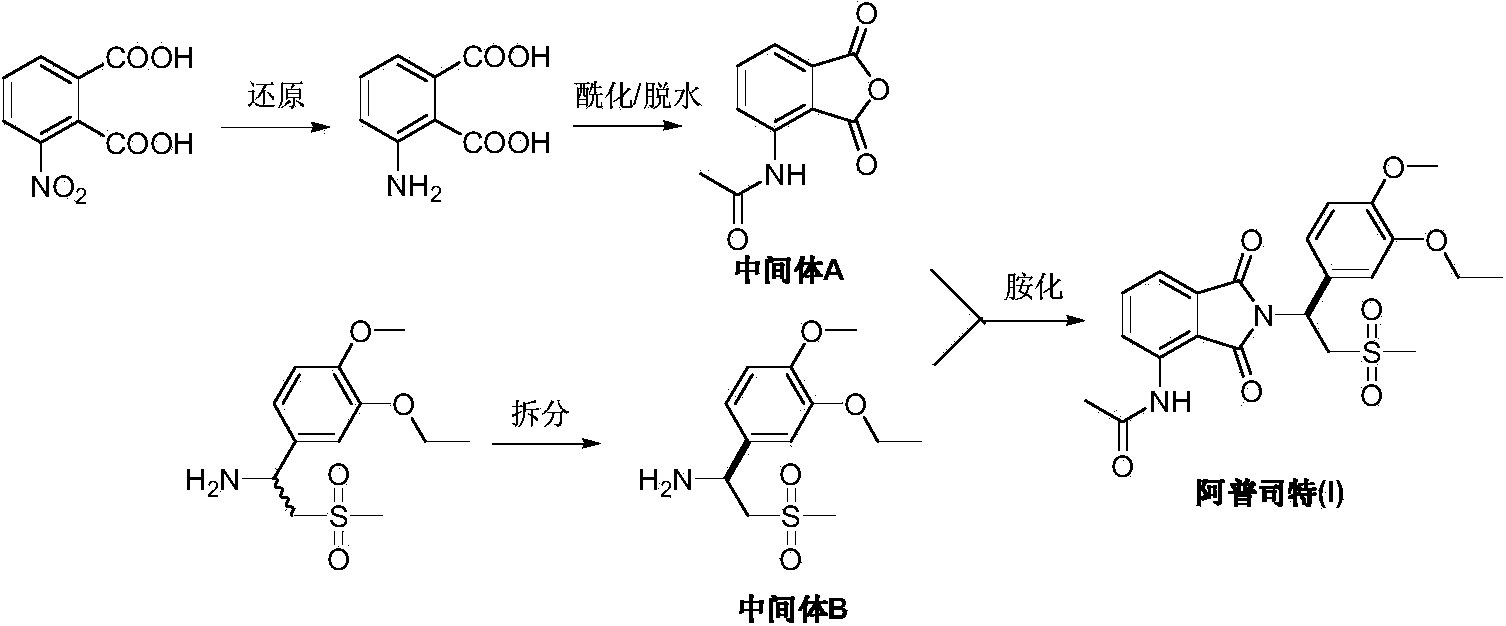
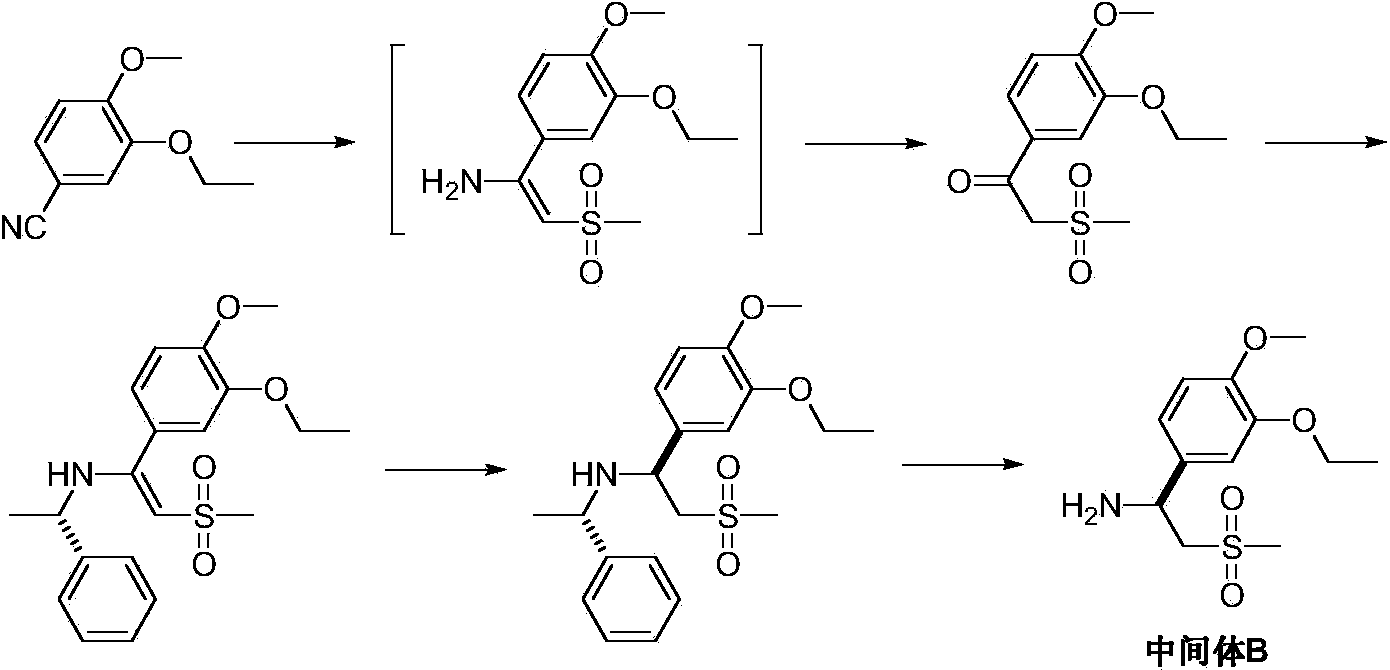
Preparation method of Apremilast
A technology of apremilast and preparation steps, which is applied in the field of preparation of apremilast, can solve the problems of harsh reaction conditions, long reaction steps, unfavorable industrialization, etc., and achieve the effects of easy availability of raw materials, promotion of development, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Add (R)-1-(α-aminobenzyl)-2-naphthol (II) (2.5g, 10mm01), triethylamine (1.2g, 12mm01) and methanol 50mL in a three-neck reaction flask, stir 3-Ethoxy-4-methoxybenzaldehyde (III) (1.8 g, 10 mmOl) was added, and after 24 hours of reaction at room temperature, a solid was formed, and the reaction was completed by TLC detection. Filtered, the filter cake was washed with cold methanol, and dried in vacuo to obtain a white solid (1R,3S)-1-phenyl-3-(3-ethoxy-4-methoxyphenyl)-2,3-dihydro- 3.7 g of 1H-naphtho[1,2-e][1,3]oxazine (IV), yield 90.0%.
Embodiment 2
[0032] Add (1R,3S)-1-phenyl-3-(3-ethoxy-4-methoxyphenyl)-2,3-dihydro-1H-naphthalene to a nitrogen atmosphere and a dry three-neck reaction flask [1,2-e][1,3]oxazine (IV) (2.1g, 5mm01) and 25mL of anhydrous-treated fresh tetrahydrofuran, add dimethyl sulfone monolithium dropwise under stirring at 0-5°C Salt (0.64g, 6.25mm01) in tetrahydrofuran solution, after dropping, raised to room temperature, stirred and reacted for 1 hour, and detected the reaction by TLC. The reaction was quenched with saturated ammonium chloride. Extracted 3 times with ethyl acetate, combined the organic phases and dried over anhydrous magnesium sulfate. Concentrated under reduced pressure, the residue was recrystallized with ethanol and water (1:1) to obtain off-white solid N-[(2S)-(1-(3-ethoxy-4-methoxyphenyl)-2- Methylsulfonylethyl)]-(1R)-(α-aminobenzyl)-2-naphthol (V) 2.2g, yield 87.1%.
Embodiment 3
[0034] Add N-[(2S)-(1-(3-ethoxy-4-methoxyphenyl)-2-methylsulfonylethyl)]-(1R)-(α- Aminobenzyl)-2-naphthol (V) (1.3g, 2.5mm01), 10% palladium carbon (65mg, 5% w / w) and methanol 50mL, according to the hydrogenation reduction operating procedure, the hydrogen pressure is controlled to be 2KG, and the temperature It is 30-40°C and reacts until no hydrogen is absorbed. Filter and recover the catalyst. The filtrate was concentrated under reduced pressure, and the residue was recrystallized from methanol to obtain (S)-2-[1-(3-ethoxy-4-methoxyphenyl)]-1-methanesulfonyl-2-ethylamine ( Intermediate B) 0.6g, yield 87.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com