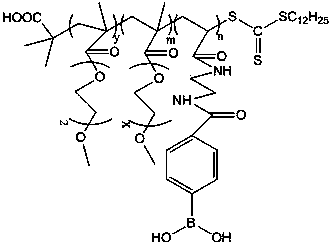
Preparation method of glucose and temperature-responsive insulin controlled release carrier
A temperature-responsive, controlled-release carrier technology, applied in the fields of polymer materials and biomedical engineering, can solve problems such as allergy, edema, control of blood sugar concentration, hypoglycemia, etc., and achieve the effect of simple and easy synthesis method and wide source.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Dissolve 28g of ethylenediamine in 150mL of 1,4-dioxane, and dissolve 13.2g of di-tert-butyl dicarbonate in 150mL of 1,4-dioxane in advance, under stirring Slowly added to the ethylenediamine solution, added dropwise for 5 hours, then reacted at room temperature for 48 hours, and obtained the product II (tert-butyl N-(2-aminoethyl)formate) through precipitation, filtration, extraction and drying; Weigh 8.5g of product II and dissolve it in 100mL of dichloromethane (DCM), add 5.6g of anhydrous Na after cooling to 0°C 2 CO3 , after stirring for a period of time, 80 mL of DCM solution of acryloyl chloride (6.0 g) was added dropwise at 0°C for 3 hours, and the reaction was continued at room temperature for 16 hours. After the reaction was completed, it was extracted, dried, and reduced pressure Distillation and other operations to obtain the product Ⅲ (N-tert-butoxycarbonyl-N 1 -acryloyl diaminoethane); Weigh 2.0g of product III and dissolve it in 10mL of DCM, add 30mL of ...
Embodiment 2
[0032] Dissolve 25g of ethylenediamine in 100mL of dichloromethane (DCM), pre-dissolve 13.0g of di-tert-butyl dicarbonate in 100mL of DCM, slowly add to the ethylenediamine solution, dropwise for 6h, Then react at room temperature for 54h, and obtain product II (N-(2-aminoethyl) tert-butyl formate) through operations such as precipitation, filtration, extraction and drying; 9.0 g of product II N-(2-aminoethyl) Dissolve tert-butyl formate in 80 mL of chloroform, add 5.3 g of anhydrous Na after cooling to 0 °C 2 CO 3 After stirring for a period of time, 60 mL of acryloyl chloride (6.0 g) in chloroform was added dropwise at 0°C for 4 hours, and the reaction was continued at room temperature for 18 hours. After the reaction was completed, it was extracted, dried, and distilled under reduced pressure. and other operations to obtain the product Ⅲ (N-tert-butoxycarbonyl-N 1 -acryloyl diaminoethane); Weigh 1.94g of product III and dissolve it in 10mL of DCM, add 28mL of trifluoroace...
Embodiment 3
[0035] Dissolve 30g of ethylenediamine in 80mL of chloroform, pre-dissolve 14g of di-tert-butyl dicarbonate in 120mL of chloroform, and slowly add it to the ethylenediamine solution under stirring, dropwise for 7h , and then reacted at room temperature for 60h, and obtained the product II (N-(2-aminoethyl) tert-butyl formate) through precipitation, filtration, extraction and drying; weighed 9.0g of the product II N-(2-aminoethyl Base) tert-butyl formate was dissolved in 80mL of chloroform, and after cooling to 0°C, 6.0g of anhydrous Na was added 2 CO 3 After stirring for a period of time, 80 mL of chloroform solution of acryloyl chloride (6.5 g) was added dropwise at 0°C for 5 hours, and the reaction was continued at room temperature for 20 hours. After the reaction was completed, it was extracted, dried, and reduced Pressure distillation and other operations to obtain the product Ⅲ (N-tert-butoxycarbonyl-N 1 -acryloyldiaminoethane); Dissolve 2.15g of product III in 10mL of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com