Penicillin G acylation enzyme mutant, and application thereof in synthesis of cephalosporin antibiotics
A technology of acylase mutants and penicillin, applied in applications, enzymes, enzymes, etc., can solve problems such as heavy computational workload, long time, and inability to achieve high activity and improved enzyme activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 Site-directed mutation
[0027] The Phe519 of penicillin G acylase was mutated to Ala, so oligo7 software was used to design blunt-end primers, and then PCR site-directed mutation was performed. This PCR mutation uses the KOD-Plus-Neo DNA polymerase kit (purchased from Toyobo Technology Co., Ltd., Shanghai). The base sequence of the primer is:
[0028] PHE TO ALA-F: 5’-GCATAACTTCTCTCACGTTGACCGTGTTAA
[0029] PHE TO ALA-ANTI: 5’-GAGTCAGAACGCAGACCAGCGTAA
[0030] The PCR reaction system is shown in Table 1.
[0031] Table 1 Site-directed mutagenesis reaction system
[0032] 10×reaction buffering
5μl
dNTPs (2mM each)
5μl
Mg 2+ (25mM)
3μl
Template
1μl
PHE TO ALA-F (10mM)
1.5μl
PHE TO ALA-ANTI (10mM)
1.5μl
KOD enzyme (1U / μl)
1μl
ddH 2 O
32μl
Total
50μl
[0033] The template is a plasmid containing wild-type penicillin G acylase gene, which was commissioned to synthesize by the company. Among them, the plasmid vector is PET-28a, and the wild-type...
Embodiment 2
[0042] Example 2 Expression and Purification of Penicillin G Acylase Mutant
[0043] 1. Expression of Penicillin G Acylase Mutant
[0044] The mutant library constructed in Example 1 was transformed into E. coli JM109 competent cells. After 10 hours, the growth of the bacteria was observed, the recombinant transformants were selected for activation, and the culture was expanded for translation and expression.
[0045] (1) Inoculate the recombinant transformant in 5ml of LB medium containing kanamycin (50μg / ml), and place it in a shaker at 37°C and 200rpm for shaking culture until the OD600 reaches about 0.4-0.5 to obtain seeds liquid.
[0046] (2) The seed solution was inoculated into the auto-induction medium at 1% of the inoculum, and cultivated at 25°C for 48 hours.
[0047] The auto-induction medium formula is: arabinose 3g / L, glucose 0.5g / L, glycerol 5g / L, peptone 10g / L, phosphate 6.8g / L, sulfate 1.2g / L, NH 4 Cl2.65g / L, MgSO 4 0.98g / L, CaCl 2 0.1g / L.
[0048] (3) The bacterial liqu...
Embodiment 3
[0056] Example 3 Application of Penicillin G Acylase Mutant in the Synthesis of Cefaclor
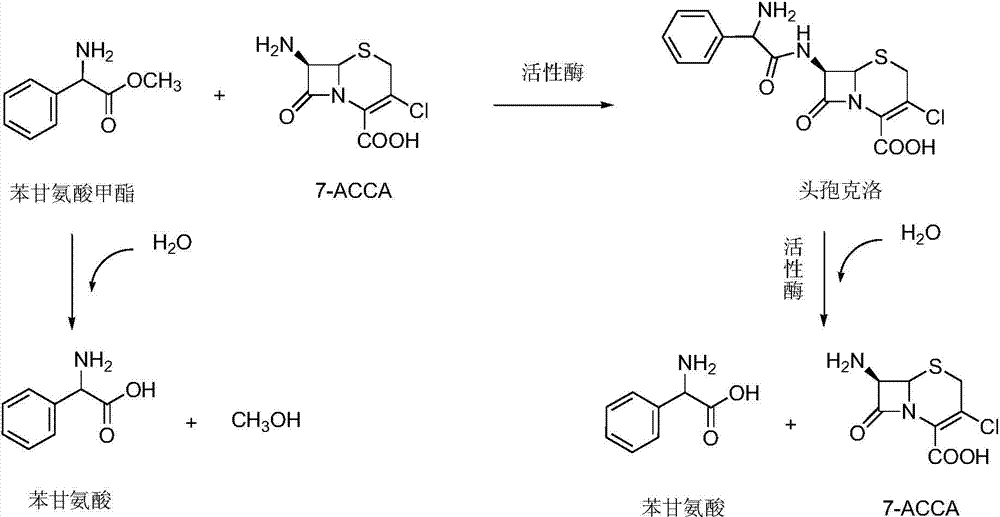
[0057] The technical scheme adopted in this embodiment is as follows figure 2 Shown.
[0058] by figure 2 The reaction formula shows that the active enzyme (penicillin G acylase) can not only condense 7-amino-3-chloro-cephalosporanic acid (nucleus 7-ACCA) and phenylglycine methyl ester (side chain) to produce products, but also decompose products The nucleus and side chains are generated, and the reversibility of the reaction is determined by the equilibrium constant of the reaction. However, phenylglycine methyl ester itself can be decomposed into phenylglycine, so it is of great significance to improve the activity of penicillin acylase.
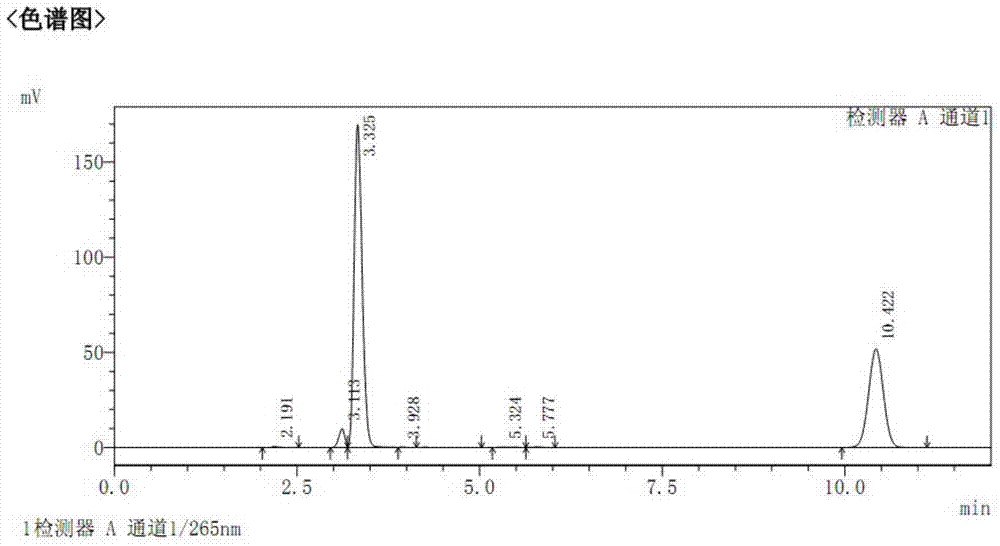
[0059] Reaction system: substrate solution (40mM 7-amino-3-chloro-cephalosporin (7-ACCA, CAS number: 53994-69-7), 40mM phenylglycine methyl ester (CAS number: 24461-61-8)) 0.525 ml, 0.1ml enzyme solution, 28℃ water bath reaction, after 20 minutes, take ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific vitality | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com