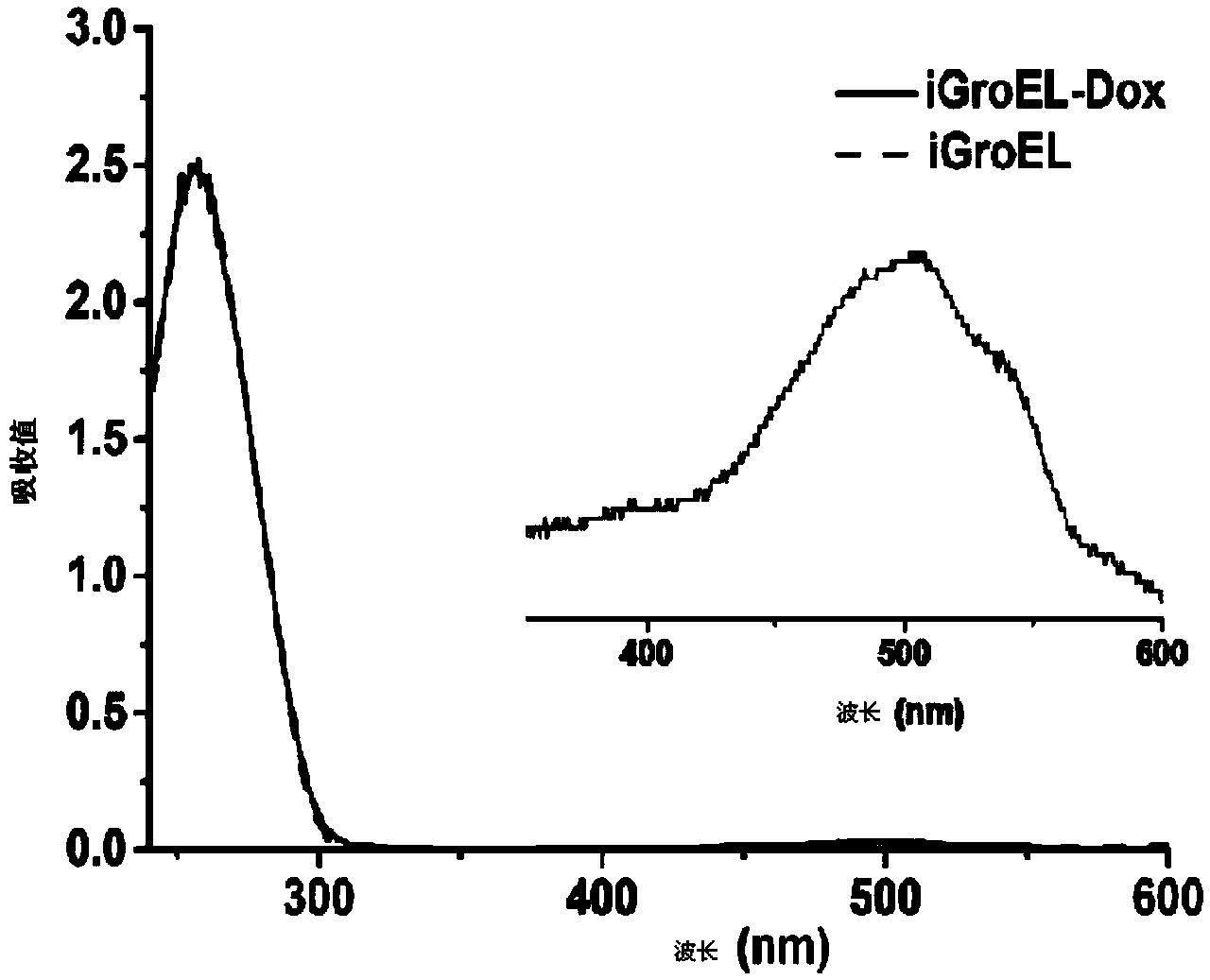
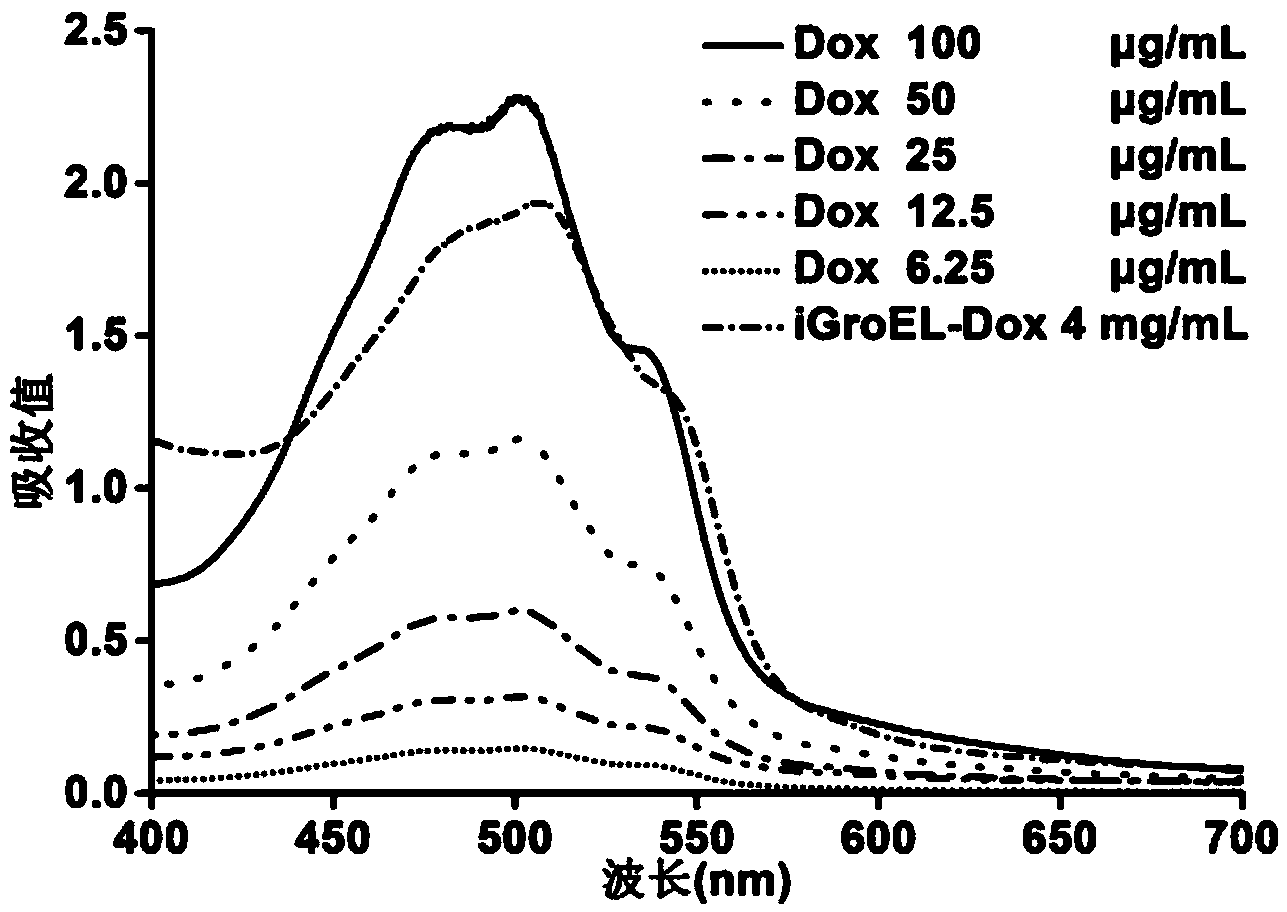
Fusion protein and encoding gene and preparation method of fusion protein as well as pharmaceutical composition and preparation method of pharmaceutical composition
A technology of fusion protein and coding gene, which is applied in the field of pharmaceutical composition and its preparation, can solve the problems of unstable drug release rate, poor drug loading stability, difficulty in carrying hydrophobic drugs, etc., and achieve good biocompatibility , High drug loading efficiency, good drug release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] The present invention also provides a method for preparing a fusion protein, which comprises expressing the coding gene of the present invention in a strain to obtain the fusion protein.
[0031] In the present invention, there are many methods for expressing the coding gene in the strain, which can be various methods conventionally used in the field. For example, the literature (Setting the chaperonin timer: A two-stroke, two-speed , protein machine. Grason JP, Gresham JS, Lorimer GH, etc., Proceedings of the National Academy of Sciences, 2008, 105(45): 17339-17344).
[0032] In the preparation method of the fusion protein of the present invention, the nucleotide sequence of the gene encoding iRGD peptide and the method of introducing the nucleotide sequence of the gene encoding the molecular chaperone GroEL into the strain can be conventionally used in the field Various methods, the invention of the present invention does not lie in this. Those skilled in the art know from...
Embodiment approach
[0049] According to a preferred embodiment of the present invention, the method for preparing the pharmaceutical composition includes contacting the pharmaceutical compound with the fusion protein of the present invention in the presence of a solvent, and the amount of the pharmaceutical compound is The amount is 0.005-0.1 parts by weight, and the contact conditions include a pH of 6-8, a temperature of 4-50°C, and a time of 12-48 hours. The materials obtained after the contact are then centrifuged, ultrafiltered and washed in sequence.
[0050] The invention also provides a pharmaceutical composition prepared by the above method of the invention.
[0051] Hereinafter, the present invention will be explained in further detail through examples. Among them, unless otherwise specified, the reagents used are all commercially available products.
[0052] In the following examples of the present invention, the used doxorubicin hydrochloride CAS number: 25316-40-9, purchased from sigma;
...
preparation example 1
[0059] This preparation example is used to obtain the iGroEL fusion protein provided by the present invention.
[0060] The amino acid sequence of the iRGD peptide and the amino acid sequence GGG connected by the bridge were inversely deduced into the gene sequence according to the genetic code to obtain the nucleotide sequence of the cDNA sequence of the iRGD peptide and the cDNA sequence of GGG, and the nucleotide sequence was combined with GroEL The primer pair (for example, the forward primer HS43 shown in SEQ ID No: 4 and the reverse primer HS45 shown in SEQ ID No: 5) are connected to obtain the amplification primer, and the amplification primer is used according to the method on molecular cloning The cDNA sequence of GroEL was amplified to obtain the recombinant DNA sequence of iGroEL fusion protein. Among them, the gene containing the iRGD peptide and the bridge-connected gene are connected with the 3'-end of the gene of the molecular chaperone GroEL sequence following PCR...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com