Artemisinin-loaded citrus pectin oral nanoparticle
A technology of nanoparticles and artemisinin, which is applied in the field of oral nanoparticles of citrus pectin, can solve the problems of good biocompatibility, instability, and no discovery.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105] The preparation of the oral nano system of embodiment 1 loading artemisinin & citrus pectin
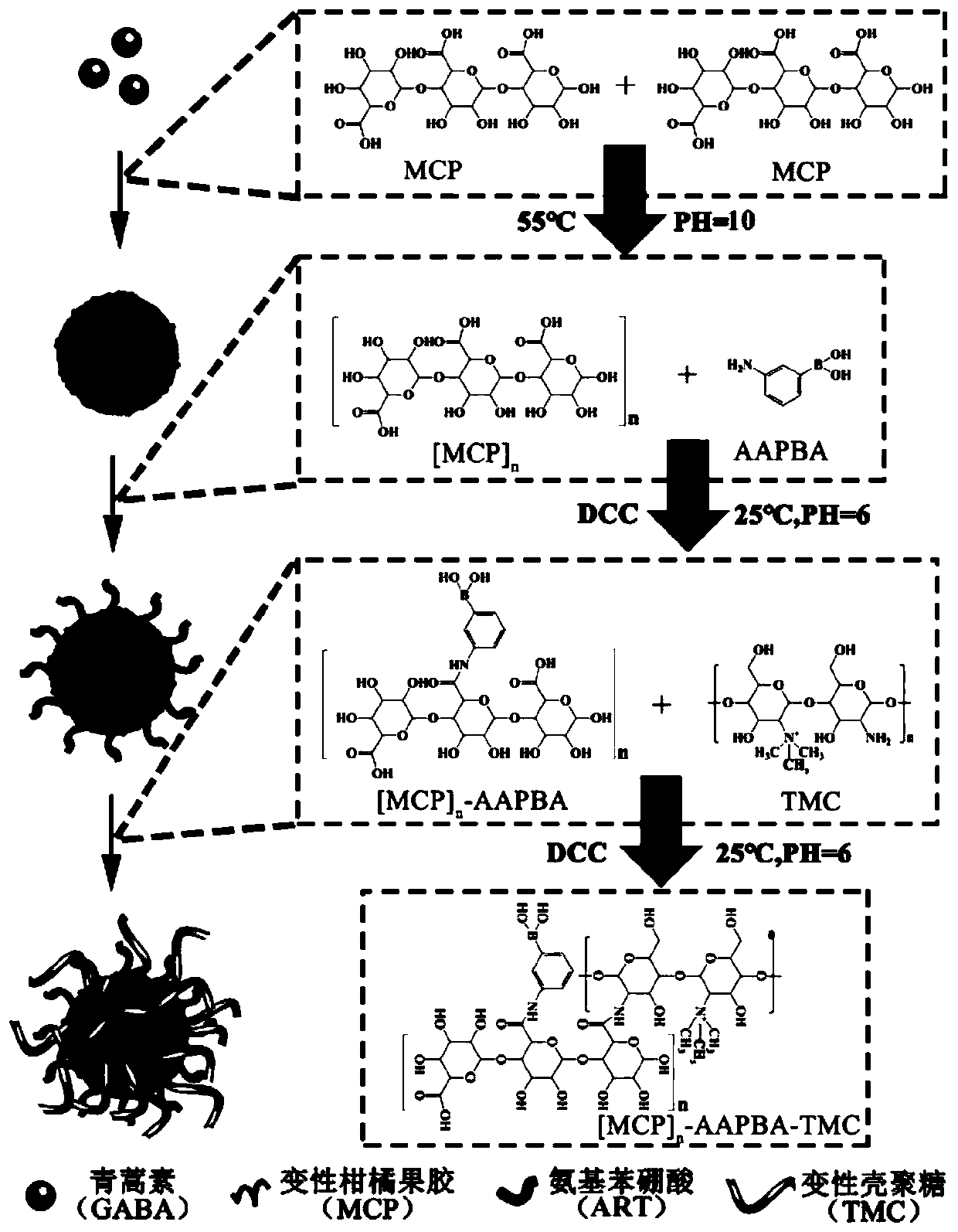
[0106] The preparation process of oral nano-particles loaded with artemisinin / citrus pectin is as follows: figure 1 Shown:
[0107] Step 1: Preparation of MCP(ART) nanoparticles
[0108] First denature the citrus pectin, place the citrus pectin in distilled water at a concentration of 1.5 mol / L, and increase its pH to 10.0 with sodium hydroxide (3 mol / L) at 55°C, and keep stirring for 1 hour , and then the solution was cooled to room temperature, its pH was adjusted to 10.0 with sodium hydroxide (3mol / L), and the denatured citrus pectin was dissolved.
[0109] Then add the artemisinin that has been dissolved in the organic solvent under the condition of 55°C, so that the final concentration of artemisinin is 1.5mol / L, stir at 600rmp for 2 hours, add calcium hydroxide to the final concentration of 0.01mol / L, Stir at 55°C and 600rmp for 1 hour, finally add sodium bicarbonate t...
Embodiment 2
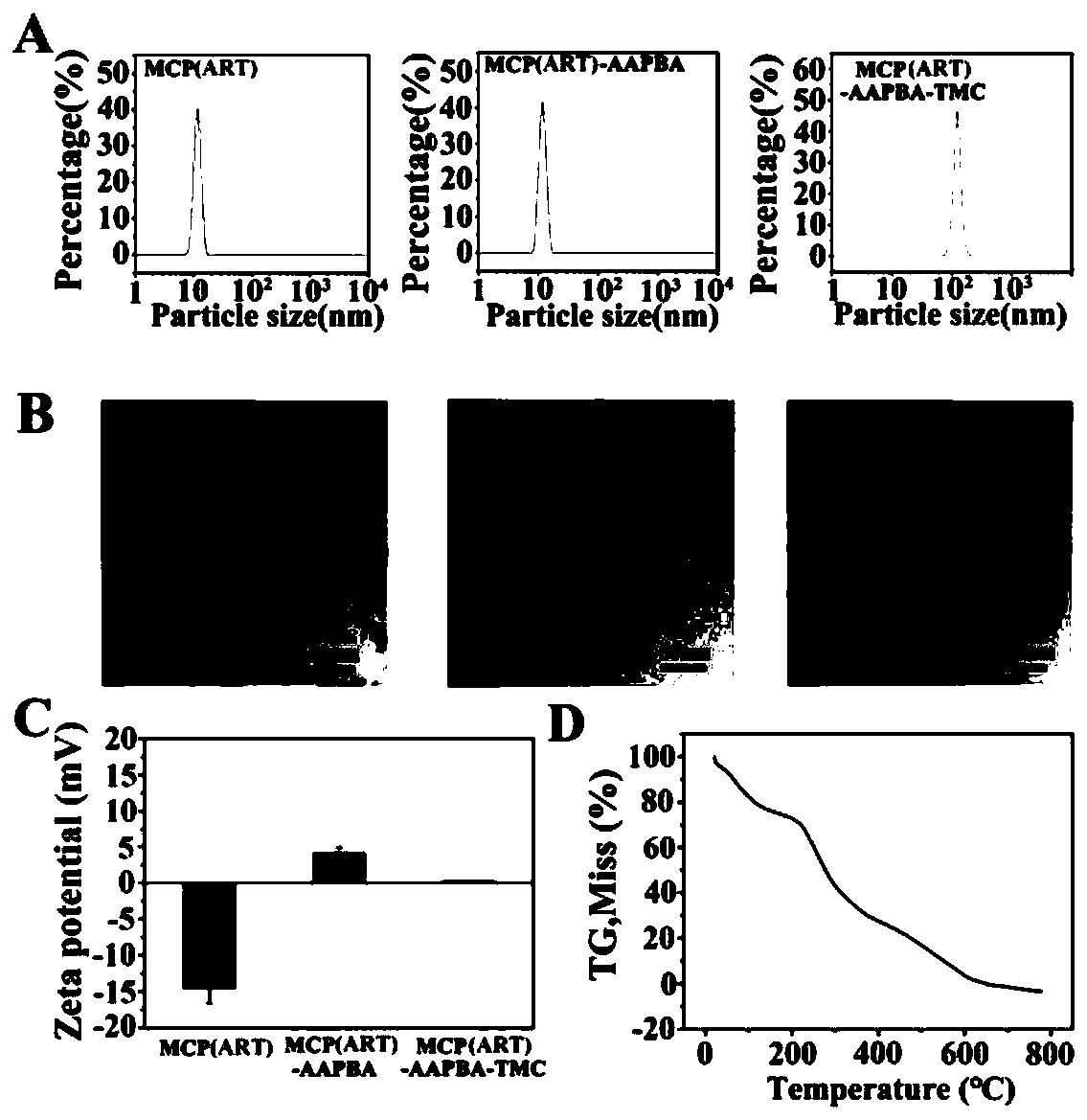
[0115] Example 2 Characterization of Oral Nanoparticles Loaded with Artemisinin & Citrus Pectin
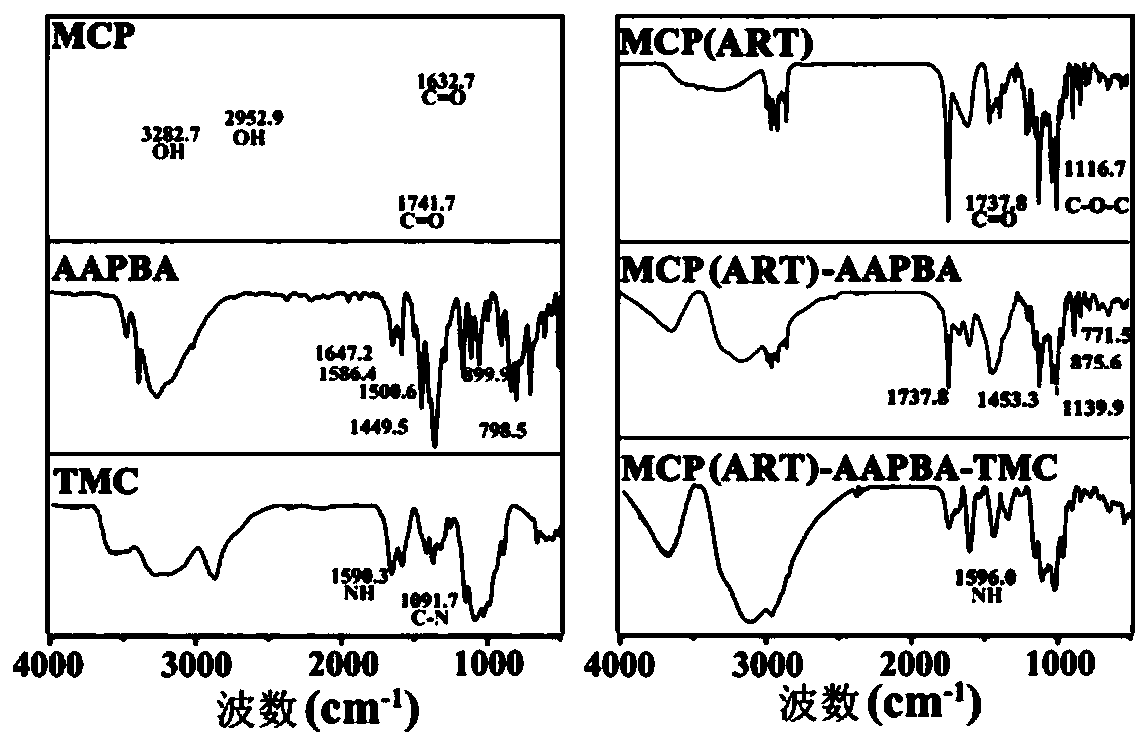
[0116] 1. Infrared spectrum detection
[0117] The raw materials MCP, AAPBA, TMC synthesized by the system and the synthetic materials MCP(ART), MCP(ART)-AAPBA and MCP(ART)-AAPBA-TMC prepared in Example 1 were detected by infrared spectroscopy.
[0118] 1. Experimental method
[0119] Freeze-dry the sample to be tested, then put it into a mortar, add a certain amount of KBr, and grind the mixture evenly until the particle size is less than 2 μm, so as to avoid the influence of scattered light, and then put it in a dryer for drying. Press the mixture into a transparent sheet with a pressure of about 10 MPa on a hydraulic press, and use a German Bruker VERTEX 33 Fourier transform infrared spectrometer to measure it.
[0120] 2. Experimental results
[0121] In order to understand the changes in the stage of forming loaded artemisinin / citrus pectin particles, the loaded artemisini...
Embodiment 3
[0165] Example 3 Cell Experiment of Oral Nanoparticles Loaded with Artemisinin & Citrus Pectin
[0166] 1. Experimental method
[0167] The biotoxicity of MCP(ART)-AAPBA-TMC oral nanotransport carrier at the cellular level will be evaluated by MTT method.
[0168] 1. Cell culture
[0169] The HepG-2 cell line was provided by Guangdong Pharmaceutical University. After the cells were proliferated and cultured to 80% in the culture flask, they were seeded on a 96-well plate at a density of 5000 / well, and cultured for 1 or 2 days for subsequent experiments. The cell culture conditions are: high-glucose DMEM medium containing 20% newborn calf serum, non-essential amino acids, 37°C, 5.0% CO 2 .
[0170] 2. Toxicity of MTT transport system
[0171]Different doses of the transport system were added to the cells being cultured, and after 24 hours, the cell viability was measured by the MTT method. That is, inoculate HepG-2 cells on a 96-well culture plate, 5000 cells / well, afte...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com