Method for synthesizing rare earth doped potassium titanate powder with photocatalytic activity
A photocatalytic activity, potassium titanate technology, applied in chemical instruments and methods, physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, etc., can solve problems such as difficult to degrade organic pollutants, and achieve Strong visible light catalytic activity, high visible light utilization, and size controllable effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
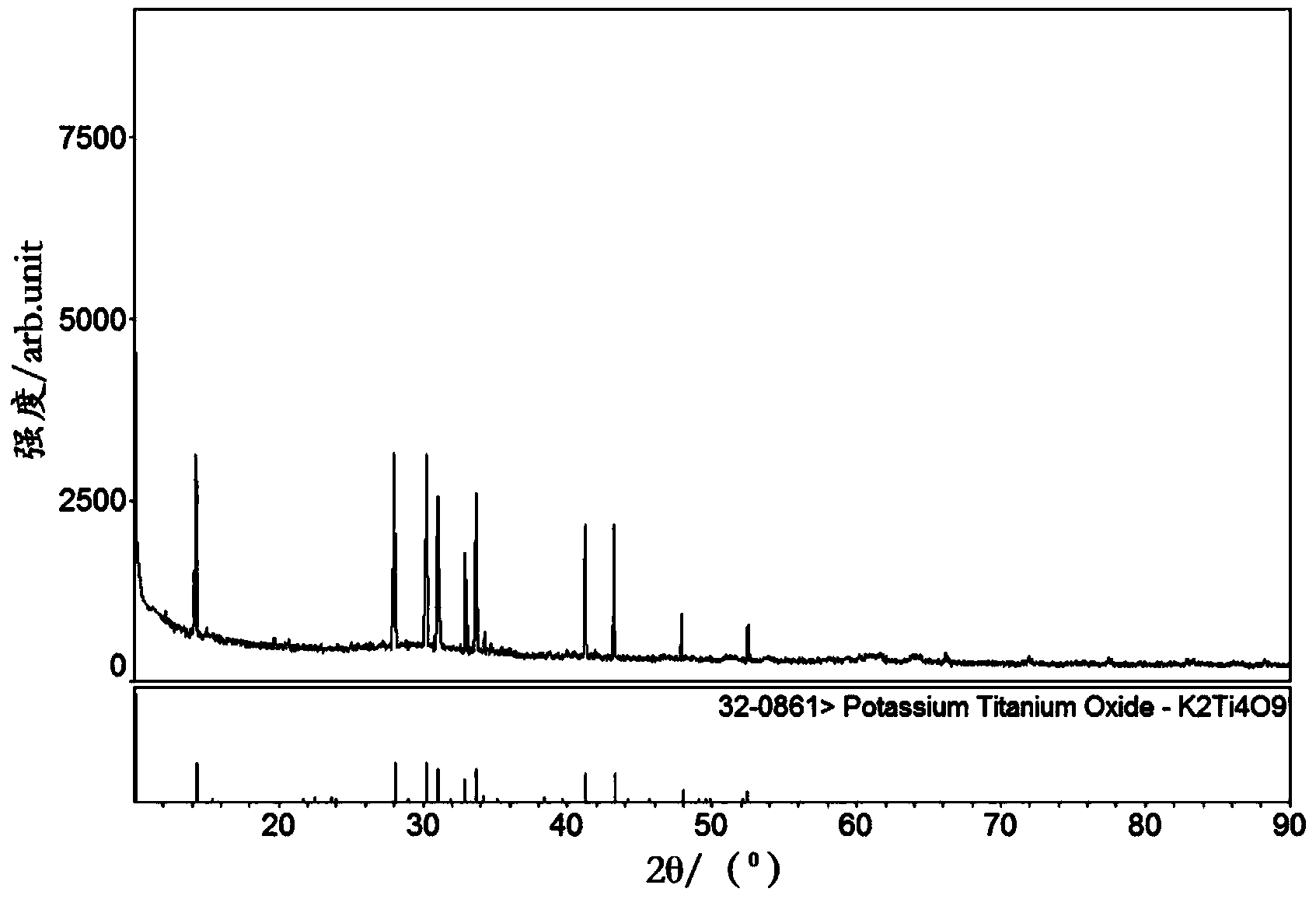
Image
Examples
Embodiment 1
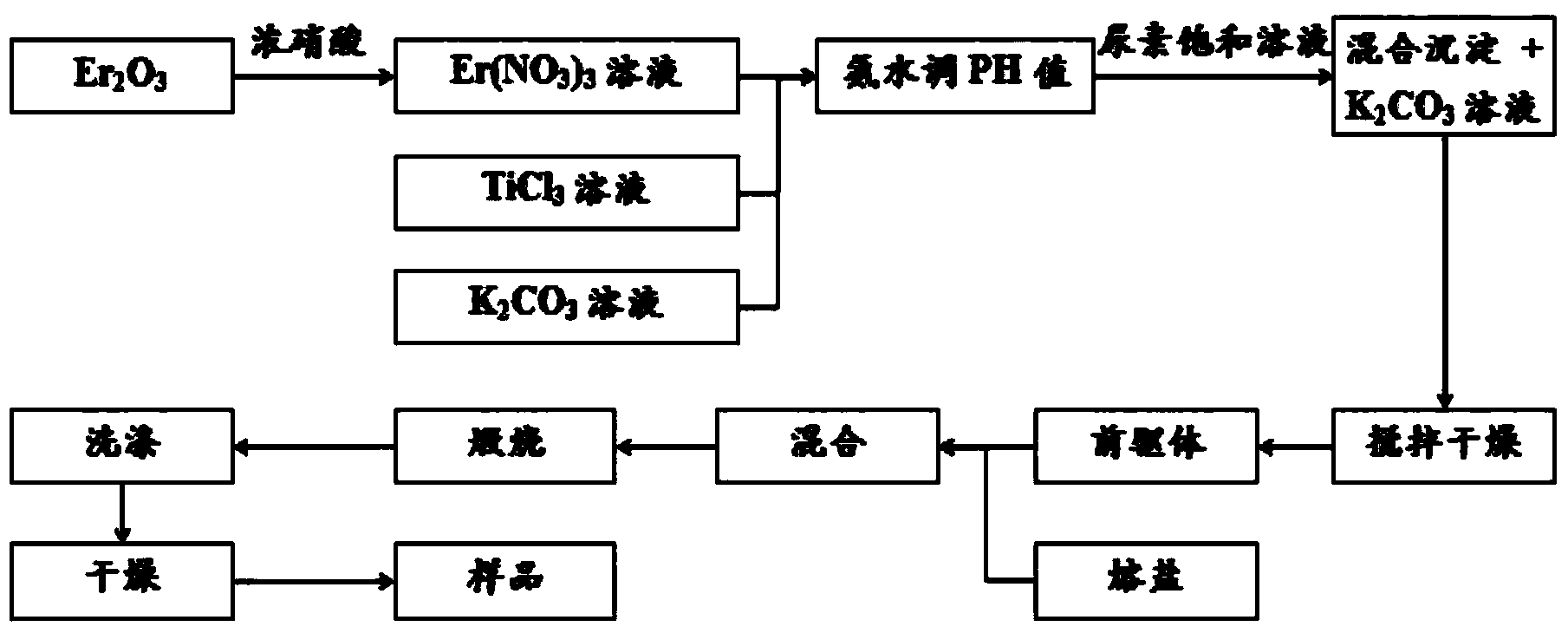
[0035] (1) Yb(NO 3 ) 3 ·5H 2 O was dissolved in distilled water to form a concentration of 0.2 mol l -1 Stable and transparent Yb(NO 3 ) 3 solution;
[0036] (2) TiCl 3 and KCl solution and Yb(NO) prepared in step (1) 3 ) 3 The solutions are mixed so that Yb in the mixed solution 3+ : Ti 3+ :K + The molar ratio is 0.5:99.5:50, and the pH value is adjusted to 7.5 with ammonia water;
[0037] (3) Add a slightly excess saturated urea solution to the mixed solution in step (2) until the pH value is 9.5, and heat to 75°C until a uniform and stable mixed precipitation liquid phase (that is, the precursor, including the mixed precipitation and KCl solution);
[0038] (4) The precursor obtained in step (3) was stirred, dried, and ground for later use, and then Na 2 CO 3 The molten salt and the precursor powder are mixed according to the mass ratio of molten salt / precursor = 4:1, dried, placed in a ceramic crucible, calcined at 1000°C, and kept at the highest temperature ...
Embodiment 2
[0041] (1) will Ce 2 o 3 Dissolve with concentrated nitric acid to form a concentration of 0.05mol l -1 Stable and transparent Ce(NO 3 ) 3 solution;
[0042] (2) will KNO 3 Solution and the Ce(NO 3 ) 3 The solution is mixed first, and then slowly dripped into the butyl titanate solution under the condition of rapid stirring, so that the Ce in the mixed solution 3+ : Ti 3+ :K + The molar ratio is 0.3:99.9:35, and the pH value is adjusted to 6.5 with ammonia water;
[0043] (3) Add a slight excess of 2mol l to the mixed solution in step (2) -1 NaOH solution to a pH value of 9.5 to form a uniform and stable mixed precipitation liquid phase (that is, the precursor, including mixed precipitation and KNO 3 solution);
[0044] (4) Stir and dry the precursor obtained in step (3), grind it for later use, and then add NaNO 3 The molten salt and the precursor powder are mixed according to the mass ratio of composite molten salt / precursor = 3:1, dried, and placed in a ceramic...
Embodiment 3
[0047] (1) First put Er 2 o 3 Dissolve with concentrated nitric acid to form 0.1mol·l -1 Stable and transparent Er(NO 3 ) 3 solution;
[0048] (2) TiCl 3 solution and K 2 CO 3 solution with Er(NO 3 ) 3 Solution according to (Er 3+ : Ti 3+ :K + ) with a molar ratio of (0.3:99.7:50) for mixing, and adjust the pH value to 7 with ammonia water,
[0049] (3) Add a slightly excess saturated urea solution to the mixed solution in step (2) until the pH value is 7.0, and heat to 75°C until a uniform and stable mixed precipitation liquid phase (that is, the precursor, including the mixed precipitation) is formed with K 2 CO 3 solution);
[0050] (4) Dry and grind the obtained precursor for later use, and then NaNO 3 The molten salt and precursor powder are mixed according to the mass ratio of molten salt / precursor = 3:1, dried, and placed in a ceramic crucible, calcined at about 700 ° C, and kept at the highest temperature for 2 hours;
[0051] (5) Finally, the crucible...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com