Preparation method of hexagonal boron nitride
A technology of hexagonal boron nitride and boron source, applied in chemical instruments and methods, nitrogen compounds, inorganic chemistry, etc., can solve the problems of complex production process, impure products, unfavorable industrialization, etc., and achieve simple process and low energy consumption. , the effect of low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
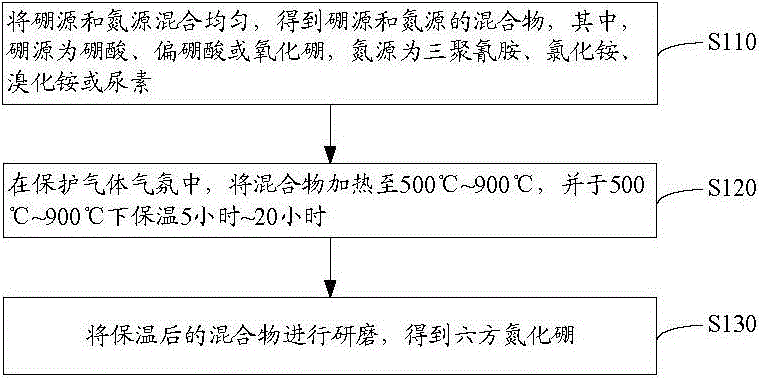
[0025] see figure 1 , The method for preparing hexagonal boron nitride according to one embodiment includes the following steps s110 to s130.
[0026] Step s110: Mix the boron source and the nitrogen source evenly to obtain a mixture of the boron source and the nitrogen source, wherein the boron source is boric acid, metaboric acid or boron oxide, and the nitrogen source is melamine, ammonium chloride, ammonium bromide or urea.
[0027] The above-mentioned boron source and nitrogen source are easily decomposed substances, so that the subsequent reaction does not need to be carried out at high temperature, and after the boron source and nitrogen source are decomposed, the boron element and nitrogen element react to form boron nitride, and there are few impurities remaining. Moreover, the price of the above-mentioned boron source and nitrogen source is relatively low, which is beneficial to reduce the preparation cost of hexagonal boron nitride.
[0028] Preferably, the molar r...
Embodiment 1
[0045] Preparation of hexagonal boron nitride
[0046] 1. Ingredients: Use an electronic balance to weigh 5g of boric acid and 20g of melamine respectively, dry the boric acid and melamine at 80°C for 24 hours, and set aside;
[0047] 2. Mixing: Using solid phase grinding, fully mix boric acid and melamine to obtain a mixture of boron source and nitrogen source, and put the mixture of boron source and nitrogen source into the crucible;
[0048] 3. Reaction process: place the crucible in a tube furnace and set the parameters of the tube furnace. Under an argon atmosphere, raise the temperature to 600°C at a rate of 8°C / min and keep it at 600°C for 12 hours. , and then grind to obtain hexagonal boron nitride.
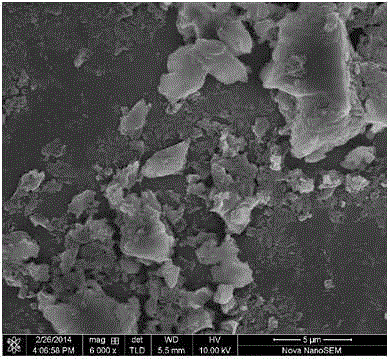
[0049] Depend on Figure 2 to Figure 5 It can be seen that the hexagonal boron nitride powder prepared in Example 1 is composed of flakes of about 1-3 microns and small nanoscale particles.
[0050] Depend on Figure 6 and Figure 7 It can be seen that compared with ...
Embodiment 2
[0053] Preparation of hexagonal boron nitride
[0054] 1. Ingredients: Use an electronic balance to weigh 3g of metaboric acid and 8g of urea, respectively, dry metaboric acid and urea at 90°C for 24 hours, and set aside;
[0055] 2. Mixing: Mix metaboric acid and urea, add 20ml of water, stir well for 2 hours, then dry to obtain a mixture of metaboric acid and urea, and put the mixture of metaboric acid and urea into a crucible;
[0056] 3. Reaction process: place the crucible in a tube furnace and set the parameters of the tube furnace. Under an argon atmosphere, raise the temperature to 800°C at a rate of 8°C / min and keep it at 800°C for 9 hours , and then grind to obtain hexagonal boron nitride.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com