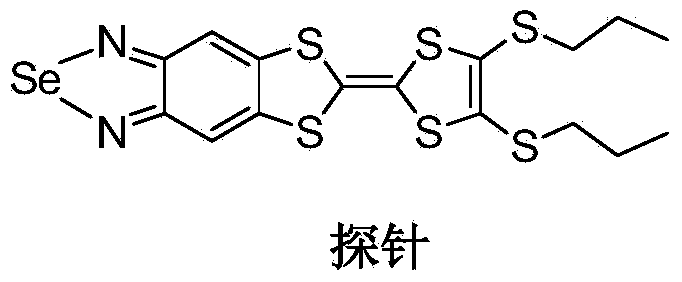
Electrochemical probe and synthesis method thereof
A synthetic method and electrochemical technology, applied in the fields of biomedicine and life sciences, to achieve the effects of good stability, good selectivity, and simple structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: the synthetic steps of electrochemical probe:
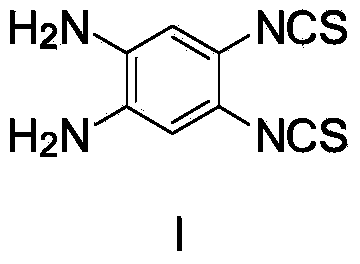
[0028] a. Add 3.5g of o-phenylenediamine, 13.0g of potassium thiocyanate, and 200mL of methanol into the container, and add bromine solution in methanol under the condition of a dry-ice acetone low-temperature bath. After the reaction is completed, stir at room temperature for 1.5h, suction filter, and filter Pour into 400mL of water, add 25mL of ammonia water to the filtrate after suction filtration, suction filtration, washing with water, and drying to obtain a yellow intermediate (I): 4,5-bis(isothiocyanato)-1,2-o-phenylenediamine , its structural formula is as follows:
[0029]
[0030] The methanol solution of bromine is 3mLBr 2 Dissolve in 50mL methanol.
[0031] b. Weigh 3.0g of the above intermediate (I) and 5.5g of sodium sulfide, add 150mL of water, heat in an oil bath at 70°C for 0.5h, add 1.5mL of carbon disulfide in an ice-water bath, after the reaction is complete, heat in an oil bath at 50...
Embodiment 2
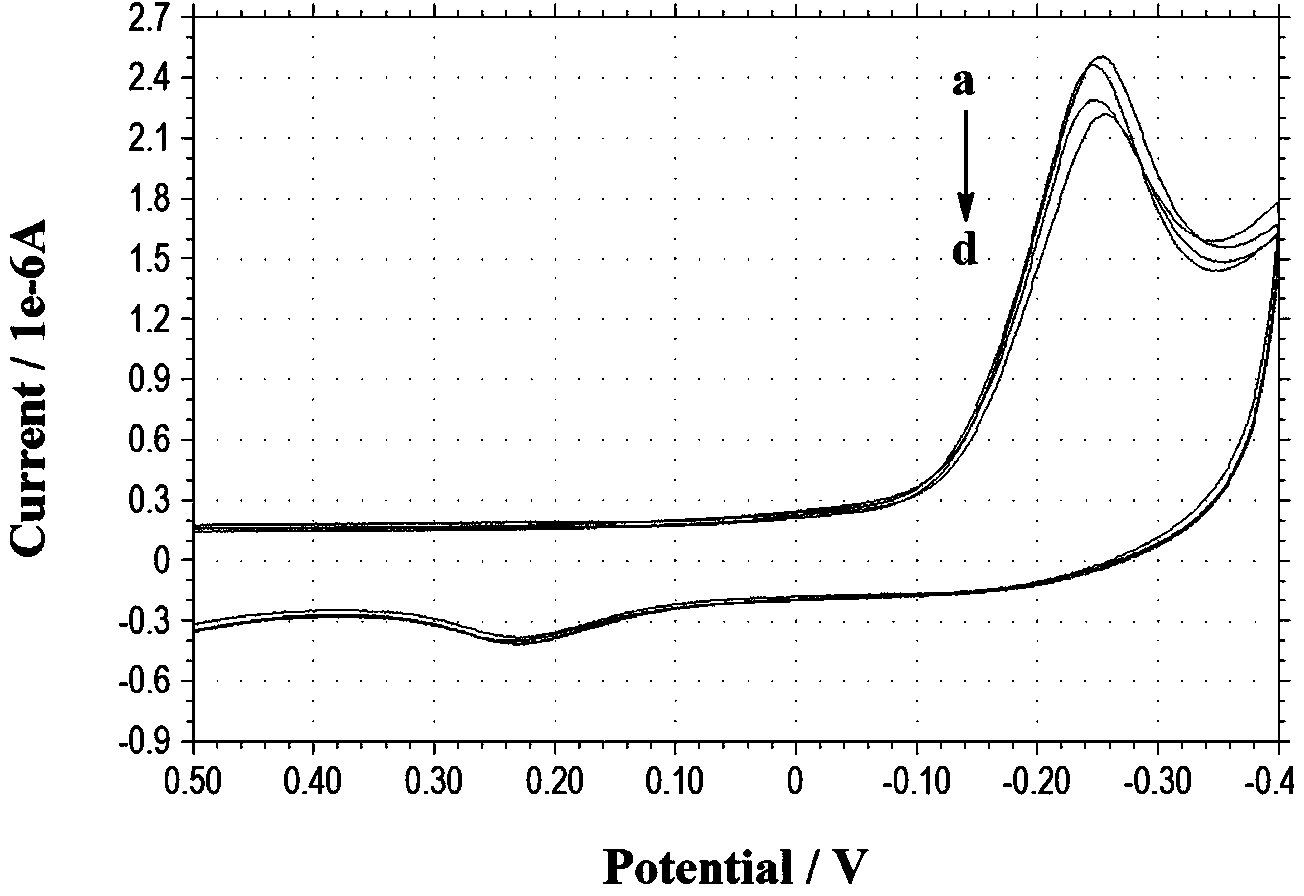
[0045] Embodiment 2: the electrochemical performance of electrochemical probe
[0046] Instruments and reagents
[0047] CHI832B electrochemical analyzer (Shanghai Chenhua Instrument Factory); all electrochemical experiments used a three-electrode system, with platinum wire as the auxiliary electrode, Ag / AgCl (saturated KCl) electrode as the reference electrode, and a bare gold electrode as the working electrode ( Diameter: 2.0mm). Differential pulse voltammetry experiments were performed in conventional electrochemical cells at room temperature. The pH value of the solution was detected with a PHS-3DpH acidity meter (compound saturated calomel electrode, Shanghai Leici Instrument Factory).
[0048] Preparation of GSH solution: Accurately weigh 3.07mgGSH in a beaker, dissolve it with a small amount of water, transfer it to a 10mL volumetric flask, and dilute with secondary water to obtain 1.0×10 -3 MGSH solution.
[0049] Preparation of electrochemical probe solution: Accu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com