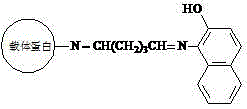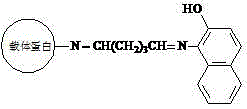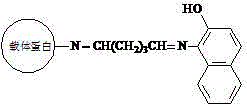Application of 1- amino-2-naphthol as hapten and corresponding artificial antigen and antibody
A technology of artificial antigen and hapten, which is applied in the field of food safety to achieve the effect of improving detection efficiency, reducing detection cost and shortening detection time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Synthesis of β-naphthol-derived dye artificial antigen
[0026] Experiment 1:
[0027] (a) Put 40mmol of 1-amino-2-naphthol in 3ml of N,N-dimethylformamide, stir and dissolve to obtain solution A; dissolve 50mg of carrier protein in 2mL of dihydrogen phosphate and hydrogen phosphate In the buffer solution of composition, obtain solution B, dihydrogen phosphate and hydrogen phosphate buffer solution preparation method are that 0.2 g potassium dihydrogen phosphate, 0.2 g potassium chloride, 1.15 g disodium hydrogen phosphate and 8.0 g sodium chloride are dissolved in 1000 mL of water, pH 7.2;
[0028] (b) Slowly add the above solution A into solution B, and stir thoroughly to obtain solution C;
[0029] (c) Add 20 μL of glutaraldehyde aqueous solution with a mass fraction of 25% to solution C dropwise, react at room temperature for 4 hours, and centrifuge at 4000 rpm for 5 minutes;
[0030] (d) Place the liquid obtained in step (c) in a dialysis bag, and dia...
Embodiment 2
[0043] Embodiment two: the preparation of monoclonal antibody:
[0044] (a) Immunize 5 Balb / C mice with the artificial antigen with the structure of formula I as the immunogen. The immunization dose is 100-300 μg / mouse. The immunization method is as follows: fully emulsify the immunogen with the same amount of complete Freund’s adjuvant, Inject subcutaneously at multiple points on the back of the neck, at intervals of 2-3 weeks, emulsify the immunogen with the same amount of Freund's incomplete adjuvant, and then boost the immunization once, a total of 6 booster immunizations;
[0045] (b) Seven days after the last immunization, the mouse with the highest titer of the above serum was selected and sacrificed by cervical dislocation. Under sterile conditions, the spleen was removed, splenocytes were separated, and mixed with mouse myeloma SP2 / 0 cells at a ratio of 10:1. Carry out fusion, use β-naphthol as a standard inhibitor to screen positive hybridoma cells, and then obtain a...
Embodiment 3
[0047] Embodiment three: performance measurement of monoclonal antibody:
[0048] (1) The lowest detection limit (LOD value) and half inhibitory amount (IC) of the antiserum 50 ) detection
[0049] The working concentration of the aforementioned β-naphthol monoclonal antibody and the artificial antigen with the structure of formula 4 was determined by square array titration. Different concentrations of β-naphthol standard substances were used as experimental solutions, and the concentrations were as follows: 0.1, 0.2, 0.5, 1, 2, 5, 10, 20, 40 (unit: ng / mL), and 6 groups of parallel experiments (n =6)
[0050] Indirect competitive enzyme-linked immunosorbent assay (ELISA): the working concentration of β-naphthol artificial antigen prepared in the above-mentioned Example 4 was used to coat the microtiter plate, overnight at 4°C or 2 hours at 37°C. Then, shake off the solution in the plate and wash it with PBST for 3-5 times, each time for 3 minutes. Add blocking solution and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com