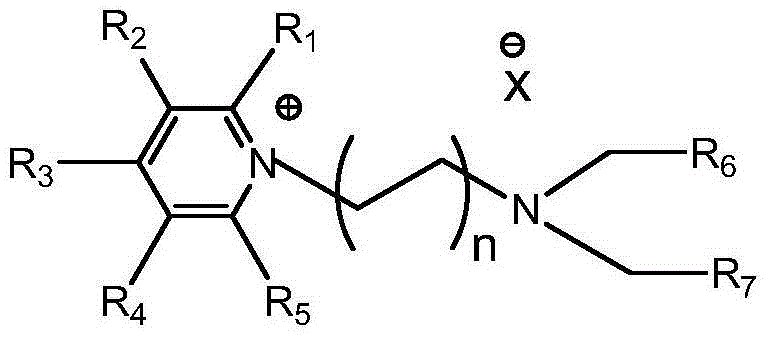
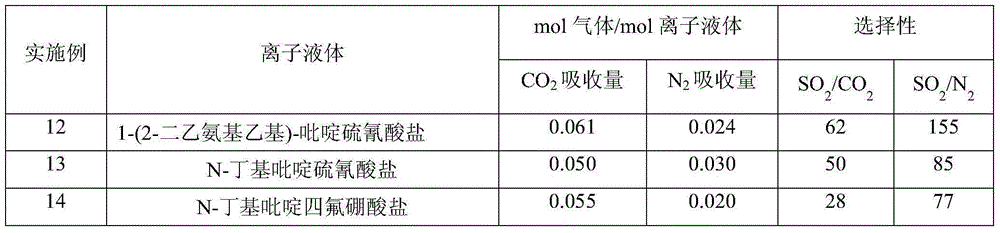
A method for capturing sulfur dioxide by functionalized ionic liquids containing tertiary amino groups and cyanopyridines
A technology containing ionic liquids and tertiary amine groups, which is applied in separation methods, chemical instruments and methods, and separation of dispersed particles. It can solve the problems of desorption requiring more heat, high biodegradability, and low synthesis cost. It is easy to achieve Effects of complete desorption, high biodegradability, and low synthesis cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] 1) Weigh 25.146g (0.096mol) of 2-diethylaminoethyl bromide hydrobromide, dissolve it in 50ml of acetonitrile and add it to a three-necked flask, mix 7.621g (0.096mol) of pyridine with 50ml of acetonitrile, Add dropwise into the three-necked bottle through the dropping funnel. After the pyridine was added dropwise, it was heated to reflux at 80°C, stirred for 12 hours, and then the reaction was terminated. The product obtained by the reaction was washed and filtered with acetone several times, and dried in vacuum at 60°C for 48 hours to obtain pure white solid 1-(2-diethylaminoethyl)-pyridinium bromide hydrobromide (31.650 g, yield 96.59%). Dissolve 1-(2-diethylaminoethyl)-pyridinium bromide hydrobromide (29.650g, 0.087mol) in 30ml of methanol, and then add equimolar hydroxide Sodium (3.487g, 0.087mol) methanol solution was stirred at room temperature for 12h, then sodium thiocyanate (7.068g, 0.087mol) was added, and stirred at room temperature for 6h. After the reacti...
Embodiment 2
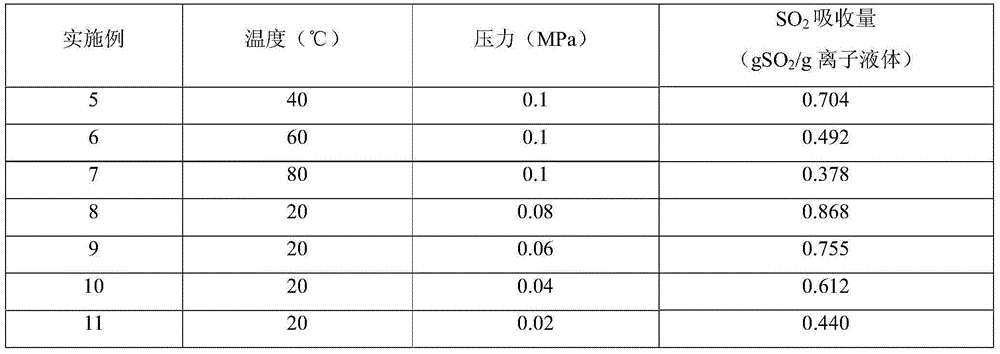
[0021] 1) In a self-made absorption bottle with an internal diameter of 3 cm, add 5.0 g of the ionic liquid 1-(2-diethylaminoethyl)-pyridine thiocyanate synthesized in 1) in Example 1, and then feed pure SO 2 For gas, the gas flow rate is 140ml / min, the temperature is 20°C, and the pressure is 0.1MPa. Weigh the absorption bottle at regular intervals until the mass does not change, and the absorption reaches equilibrium in about 90 minutes. Calculate the SO in the ionic liquid 2 The absorption capacity is 1.060gSO 2 / g ionic liquid (3.958molSO 2 / mol ionic liquid).
[0022] 2) In a self-made absorption bottle with an inner diameter of 3 cm, add 5.0 g of ionic liquid N-butylpyridine thiocyanate, and then feed pure SO 2 For gas, the gas flow rate is 140ml / min, the temperature is 20°C, and the pressure is 0.1MPa. Weigh the absorption bottle at regular intervals until the mass does not change, and the absorption reaches equilibrium in about 90 minutes. Calculate the SO in the i...
Embodiment 3
[0025] 1) In a self-made absorption bottle with an inner diameter of 3cm, add 2) ionic liquid 1-(2-diethylaminoethyl)-pyridine dicyandiamide salt synthesized in 5.0g embodiment 1, then pass into pure SO 2 For gas, the gas flow rate is 140ml / min, the temperature is 20°C, and the pressure is 0.1MPa. Weigh the absorption bottle at regular intervals until the mass does not change, and the absorption reaches equilibrium in about 90 minutes. Calculate the SO in the ionic liquid 2 The absorption capacity is 0.972gSO 2 / g ionic liquid (3.753molSO 2 / mol ionic liquid).
[0026] 2) In a self-made absorption bottle with an inner diameter of 3 cm, add 5.0 g of ionic liquid N-butylpyridine dicyandiamide salt, and then feed pure SO 2 For gas, the gas flow rate is 140ml / min, the temperature is 20°C, and the pressure is 0.1MPa. Weigh the absorption bottle at regular intervals until the mass does not change, and the absorption reaches equilibrium in about 90 minutes. Calculate the SO in th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com