Preparation method of CF2O-contained monomer liquid crystal compound
A technology of difluoromethoxy ether and liquid crystal compounds, which is applied in the preparation of organic compounds, chemical instruments and methods, liquid crystal materials, etc., can solve the problems of low price, harsh reaction conditions and high price.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0082] The invention provides a bridge containing difluoromethoxyether (CF 2 O) the preparation method of the monomer liquid crystal compound, specifically refers to the compound of formula V structure,
[0083]
[0084] Wherein said R is selected from C1-C9 substituted or unsubstituted alkyl groups, alkoxy groups, ester groups or combinations thereof; preferably, said C1-C9 substituted or unsubstituted alkyl groups are selected from CH 3 , C 2 h 5 , C 3 h 7 , C 4 h 9 , C 5 h 11 , or CH 2 OCH 3 ; Alkoxy group is selected from OR', wherein said R' is C1-C8 alkyl, and said ester group is selected from COOR "or CH 2 COOR" ester group, wherein said R" is a C1-C8 alkyl group.
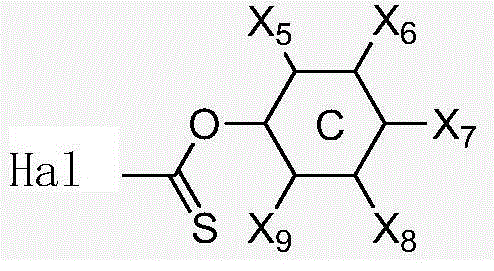
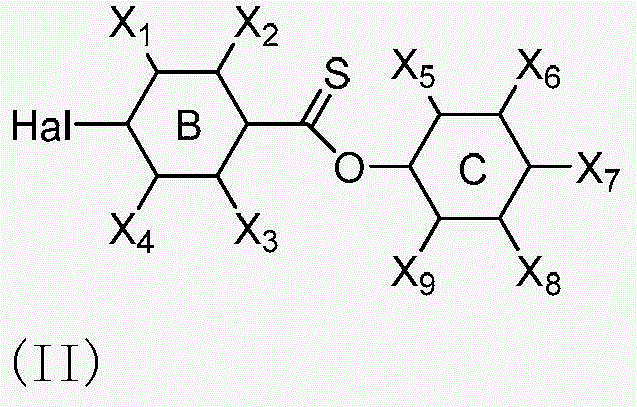
[0085] which stated selected from phenyl, cyclohexyl and derivatives thereof or combinations thereof.
[0086] which stated selected from phenyl, cyclohexyl or combinations thereof, wherein X 1 、X 2 、X 3 、X 4 Selected from F, Br, Cl or H and alkoxy or ester group; alkoxy group is selec...
Embodiment 1
[0125] Preparation of O-3,4,5-trifluorobenzene-2,6-difluoro-4-bromobenzenethiocarbamate
[0126] Under the protection of nitrogen, add 61.8g of 3,5-difluorobromobenzene and 100mL THF into a 1L four-necked bottle, cool the system to -50°C, add 359.8g of 10% LDA (lithium diisopropylamide) dropwise, and drop- Stir at 50°C for 1 hour, dissolve 34.9g of zinc chloride in 200mLTHF and add to the system and stir for 30min; add 21.6g of copper chloride and 86.8g of O-3,4,5-trifluorobenzene-chlorothioformate into 1L Add 200mL THF to the mouth bottle, add the prepared zinc reagent to the reaction system at 20°C, there is obvious exotherm, react for 4 hours after dripping, add 250mL of 10% hydrochloric acid to the system, fully acidify the system to pH<1, and let stand to separate layer, the aqueous phase was extracted twice with 100 mL of tertiary methyl ether and the organic phase was precipitated to obtain 130.5 g of a yellow crude product, and recrystallized from methanol to obtain 10...
Embodiment 2
[0130] Preparation of O-3,4,5-trifluorobenzene-2,3-difluoro-4-chlorobenzenethiocarbamate
[0131] Under nitrogen protection, add 110.9g of 2,3-difluoro-4-chloroiodobenzene, 52.5g of zinc powder and 440mL THF into a 1L four-necked bottle, and prepare zinc reagent by reflux reaction; mix 21.6g of cuprous cyanide with O-3 , Add 109.6g of 4,5-trifluorobenzene-chlorothioformate into a 1L four-necked bottle and add 200mL THF. Add the prepared zinc reagent into the reaction system at 20°C. There is obvious exotherm, and react for 4 hours after dropping . Add 250mL of water to the system, let stand to separate the layers, extract the water phase twice with 100mL of tertiary methyl ether and combine the organic phase for desolventization to obtain 139.8g of the yellow crude product, 115.8g of the target product was obtained by methanol recrystallization, and the content detected by gas chromatography was 98.3 %, the yield is 84.7%.
[0132] h 1 NMR (300MHz, CD 3 Cl): δ6.81-6.91(m,2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com