Polypeptide polymer having antitumor activity and its preparation method and use
A peptide polymer and polymer technology, applied in the field of peptide polymer with anti-tumor activity and its preparation, can solve the problems of low bioavailability, short half-life, and difficulty in entering cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1: with R 1 Polyethylene glycol with a structure of 10 repeating units as an example to prepare polypeptide polymers
[0057] 1. First introduce the synthesis method of polypeptide polymer:
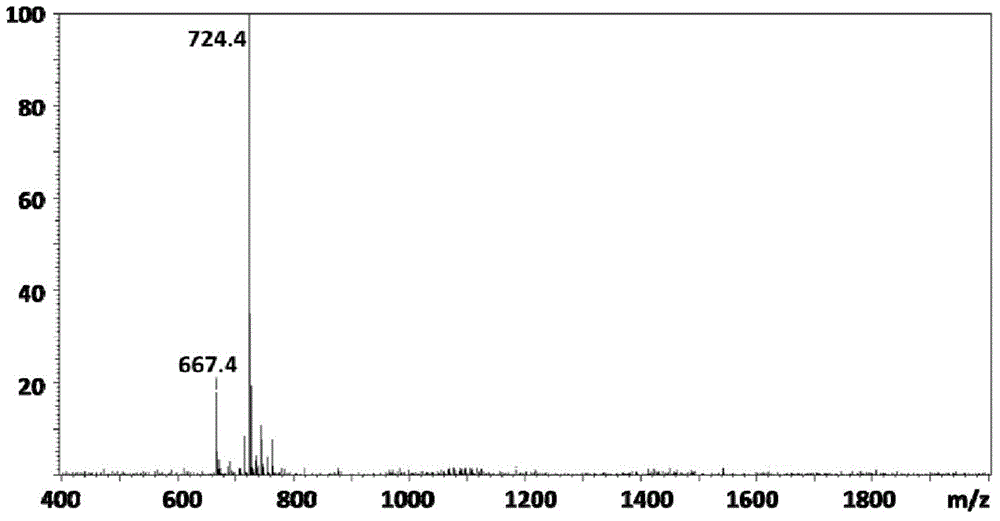
[0058] The 10mM (total amount) peptide molecule CCGGGRGD (targeting peptide) and CCGGGKLAKLAKKKLAKLAK (therapeutic peptide) were mixed in different molar ratios (100:0, 90:10, 80:20, 70:30, 60:40, 50:50, 40 :60, 30:70, 20:80, 10:90, 0:100) were dissolved in water and placed in a reaction vessel, 2 mL of phosphate buffer solution with pH 7.4 was added, stirred and dissolved, and 10 mM phosphate buffer solution with a molecular weight of 575 was added Polyethylene glycol diacrylate, a sealed system, nitrogen gas for 20 minutes, and a constant temperature of 37 ° C for 3 days; the reacted solution was added to a dialysis bag, dialyzed for 24 hours, and freeze-dried to obtain a white powdery solid. By gel permeation chromatography analysis, the molecular weight of the polymer...
Embodiment 2
[0061] Example 2: with R 1 Preparation of Polypeptide Polymers with Methylene Amide as an Example
[0062] 1. First introduce the synthesis method of polypeptide polymer:
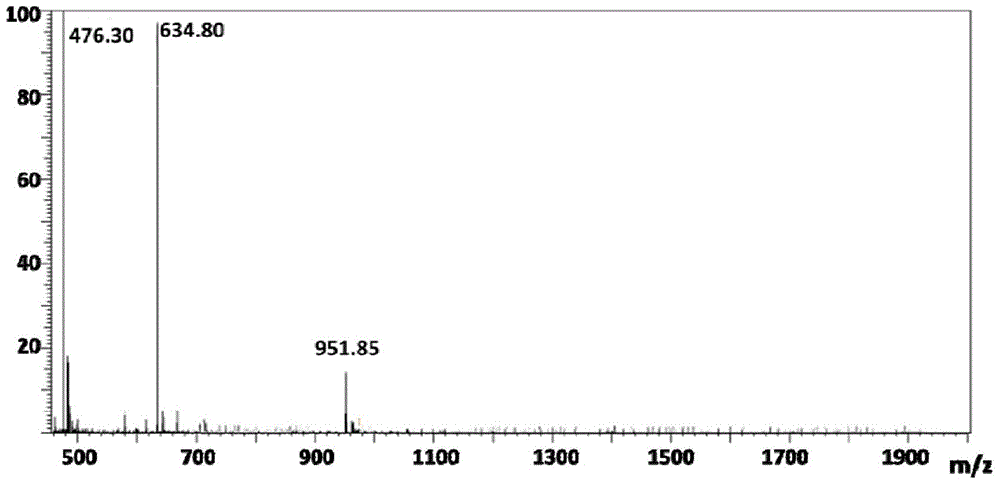
[0063] The 10mM (total amount) peptide molecule CCGGGRGD (targeting peptide) and CCGGGKLAKLAKKKLAKLAK (therapeutic peptide) were mixed in different molar ratios (100:0, 90:10, 80:20, 70:30, 60:40, 50:50, 40 :60, 30:70, 20:80, 90:10, 0:100) dissolved in water, and placed in a reaction vessel, added 20mM sodium bicarbonate, stirred to dissolve, then added 10mM N,N'-methylene Bisacrylamide, sealed system, nitrogen gas for 20 minutes, constant temperature 37 ° C reaction for 3 days; the reaction solution was added to a dialysis bag, dialyzed for 24 hours, and freeze-dried to obtain a white powdery solid. Through gel permeation chromatography analysis, the molar ratio of targeting peptide and therapeutic peptide is 10:90, the polymer molecular weight is 31200, and the dispersion degree is 1.58.
[0064] 2. Pe...
Embodiment 3
[0066] Example 3: with R 1 Preparation of Polypeptide Polymer with Structure 1,6-Hexanediol as an Example
[0067] Dissolve 10mM (total amount) polypeptide molecules CCGGGRGD (targeting peptide) and CCGGGKLAKLAKLAKKLAKLAK (therapeutic peptide) in dimethyl sulfoxide at different molar ratios, and place them in a reaction vessel, add 20mM triethylamine, stir to dissolve, Add 10mM 1,6-hexanediol diacrylate, seal the system, pass nitrogen gas for 20 minutes, and react at a constant temperature of 30°C for 3 days; put the reacted solution into a dialysis bag, dialyze for 24 hours, and freeze-dry to obtain a white powdery solid . Through gel permeation chromatography analysis, the molar ratio of targeting peptide and therapeutic peptide is 10:90, the molecular weight of the polymer is 35600, and the dispersion is 1.42.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com