Porous cobalt oxide nanowire, and preparation method and application thereof
A technology of cobalt trioxide nanometer and cobalt trioxide nanometer, which is applied in the field of nanomaterials, can solve the problems of surface conductivity change, low response sensitivity, high working temperature, etc., and achieve the effect of large choice, high gas sensitivity response selectivity and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1: Using cobalt chloride as cobalt source
[0051] Dissolve and disperse 1.25mmol cobalt chloride and 1.25mmol urea in 40ml deionized water as solution A;
[0052] Dissolve and disperse 1.25 mmol of sodium fluoride in 40 ml of deionized water as solution B.
[0053] After mixing solution A and solution B uniformly, they were transferred to a 100ml hydrothermal kettle and allowed to stand at 120°C for 12 hours.
[0054] After natural cooling, the light pink flocculent precipitated product obtained after the reaction was separated by centrifugation, washed several times with water, and dried in an oven at 100°C. The obtained dry powder was slowly heated to a temperature of 1°C / min in a muffle furnace The product was calcined at 300°C for 3 hours at a constant temperature and cooled naturally.
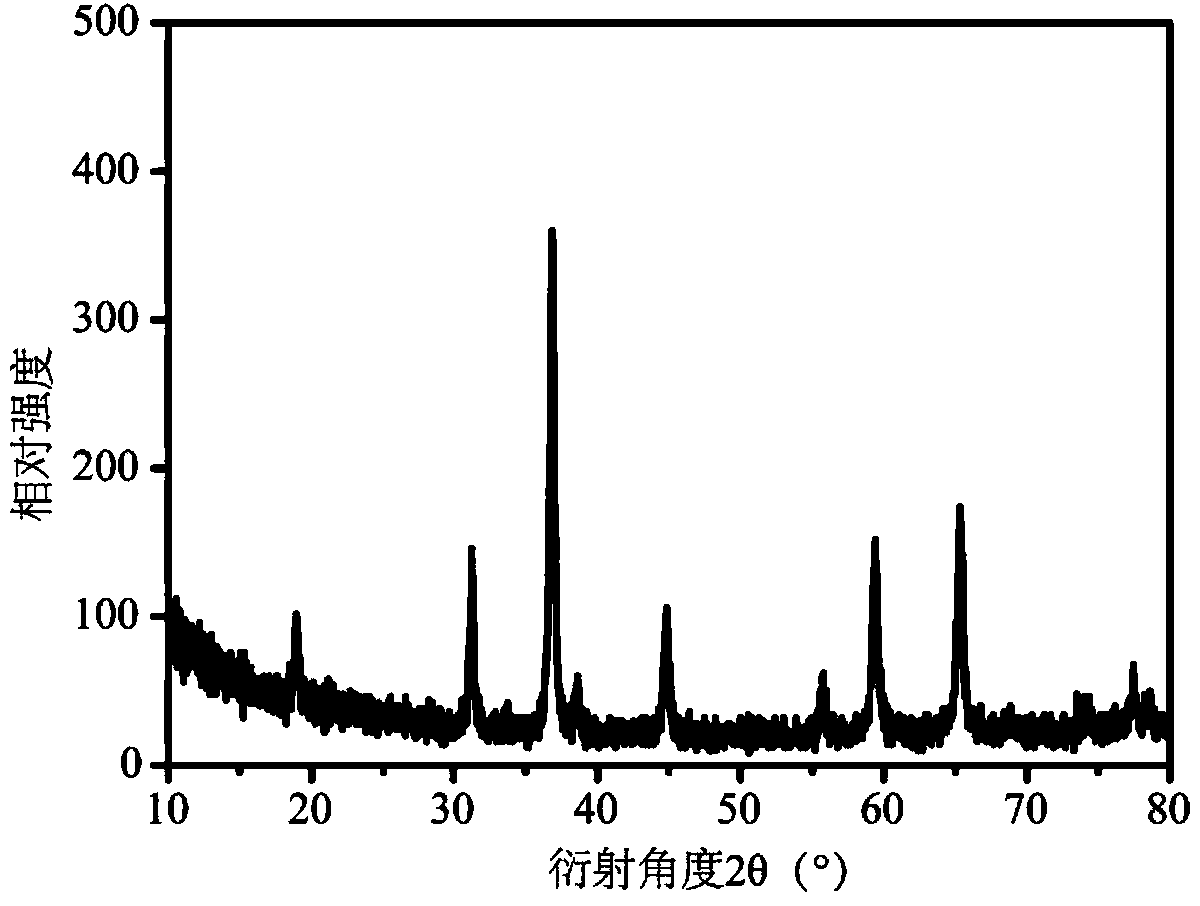
[0055] The product was identified as cubic cobalt tetroxide by X-ray powder diffractometer (such as figure 1 Shown);
[0056] Use SEM (such as figure 2 Shown) and TEM (as image 3 ...
Embodiment 2
[0061] Example 2: Using cobalt nitrate as cobalt source
[0062] Dissolve and disperse 1.25mmol cobalt nitrate and 1.25mmol urea in 40ml deionized water as solution A;
[0063] 1.25mmol sodium fluoride was dissolved and dispersed in 40ml deionized water as solution B.
[0064] After mixing solution A and solution B uniformly, they were transferred to a 100ml hydrothermal kettle and reacted hydrothermally at 150°C for 2 hours. After natural cooling, the pale pink flocculent precipitated product obtained after the reaction was separated by centrifugation, washed several times with water, and dried in an oven at 100°C. The resulting dry powder was slowly heated to a temperature of 2°C / min in a muffle furnace After being calcined at 350°C for 2 hours at a constant temperature, the product was naturally cooled to obtain the product.
[0065] The X-ray powder diffraction spectrum is the same as in Example 1 (e.g. Picture 11 Shown);
[0066] Use SEM and TEM (respectively as Picture 12 with...
Embodiment 3
[0069] Example 3: Inorganic sodium carbonate as alkali source
[0070] Dissolve 1.25mmol cobalt chloride and 1.25mmol anhydrous sodium carbonate in 40ml deionized water as solution A;
[0071] 1.25mmol sodium fluoride was dissolved and dispersed in 40ml deionized water as solution B.
[0072] After mixing solution A and solution B uniformly, they were transferred to a 100ml hydrothermal kettle and allowed to stand at 140°C for 4 hours. After natural cooling, the light pink flocculent precipitated product obtained after the reaction was separated by centrifugation, washed several times with water, and dried in an oven at 100°C. The obtained dry powder was slowly heated to a temperature of 4°C / min in a muffle furnace After being calcined at 400°C for 1 hour at a constant temperature, the product was naturally cooled to obtain the product.
[0073] The X-ray powder diffraction spectrum is the same as in Example 1 (e.g. Figure 16 Shown);
[0074] Use SEM and TEM (respectively as Figure ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| electrical resistivity | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com