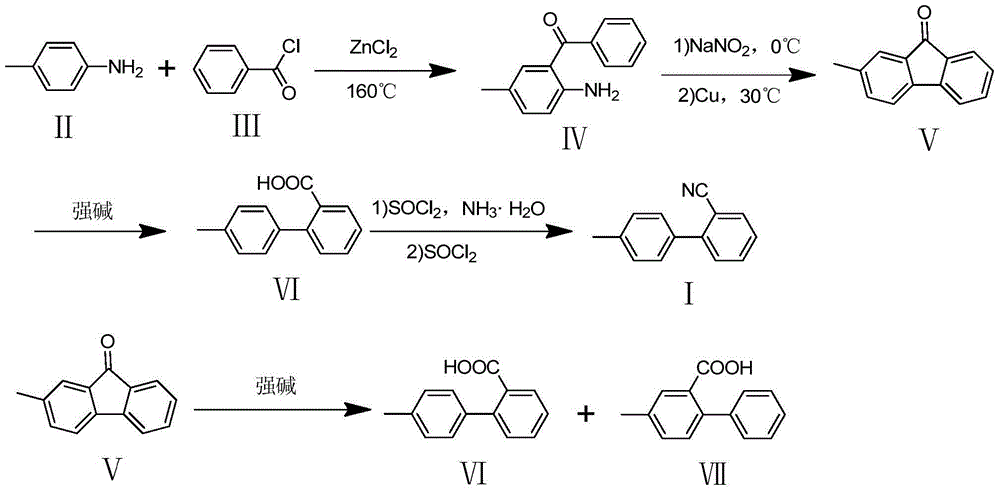
Synthetic method of 2-cyano-4'-methylbiphenyl
A technology of sartan biphenyl and its synthesis method, which is applied in the direction of condensation preparation of carbonyl compounds, carboxylic acid amide dehydration preparation, organic chemistry, etc., which can solve the problems of inability to carry out large-scale industrial production, increase the difficulty of separation and purification, and low total reaction yield and other issues, to achieve the effect of improving the utilization rate of atoms, facilitating production management, and easily obtaining raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0017] Preparation of 2-amino-5-methylbenzophenone (Ⅳ)
[0018] Put benzoyl chloride (133.6mL, 1.15mol) in a reaction flask, stir, heat up to 120°C, slowly add p-toluidine (50.00g, 0.46mol), heat up to 140°C after the addition, and add no Water ZnCl2 (79.06 g, 0.58 mol), the temperature was raised to 160 ° C for 3 hours, the temperature was lowered to 100 ° C, 200 mL of water was added, stirred, and the water layer was poured out after cooling. Add 150mL of 70% sulfuric acid to the residue in the reaction bottle, stir at 140°C for 2 hours, pour the reaction solution into a large amount of water after cooling, neutralize the reaction solution with ammonia water, extract with ethyl acetate, and remove the ethyl acetate by rotary evaporation. The solid was recrystallized from 95% ethanol to obtain a yellow solid (79.70 g, yield 82.2%).
[0019] 1H NMR (400MHz, CDCl3, ppm) δ: 7.64(d,1H),7.54(t,1H),7.47(t,2H),7.23(s,1H),7.13(d,1H),5.91(s, 2H), 2.18(s, 3H).
[0020] Preparation o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com