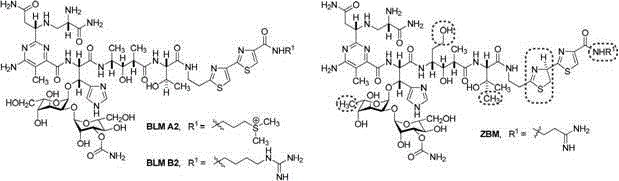
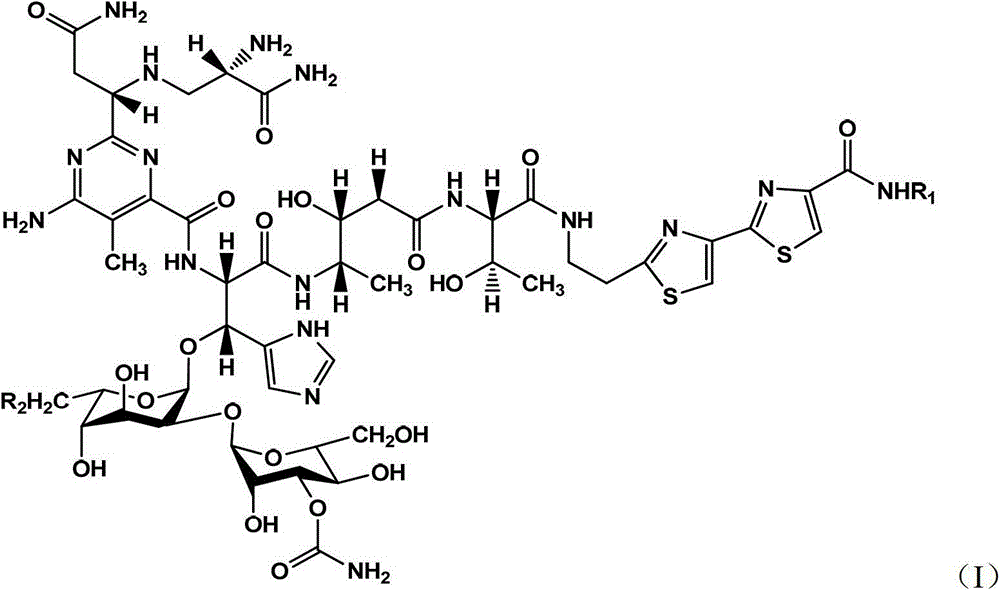
Microbial fermentation preparation method of bleomycin derivatives
A bleomycin family and microbial fermentation technology, applied in the field of biomedicine, can solve the problems of low fermentation yield and low efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Both Streptomyces chrysogenum SB9001 and the genetically engineered mutant strain obtained in the experiment were cultured on ISP4 agar solid medium at 30°C to obtain spores. Streptomyces verticillium ATCC15003 was grown in TSBY liquid medium containing 0.5% glycine at 28°C and 250 rpm to obtain genomic DNA. Escherichia coli was grown in LB liquid medium at 37°C and 250rpm for daily plasmid cloning and BAC library establishment.
[0055] 1) Construction of Streptomyces chrysanthemum SB9025 mutant strain
[0056] A 10kb DNA fragment containing the complete zbmVIII gene was isolated from the cosmid containing a part of the zobermycin biosynthetic gene cluster zbm, and cloned into the Litmus 28 plasmid through BamHI and BglII restriction sites superior. The resulting plasmid was digested with Sbf1 to remove the insert fragment with a size of about 3.7 kb, and then the plasmid was self-ligated to complete the knockout of the zbmVIII gene. The modified DNA insert was dige...
Embodiment 2
[0063] Separation, purification and detection of novel bleomycin analogs BLM S (Tianci 102, compound 1) and 6’-dehydroxy-BLM S (Tianci 103, compound 2)
[0064] The supernatant solution obtained after the fermentation broth was centrifuged was detected and analyzed by high-performance liquid phase (HPLC). The mobile phase A was 99.9% deionized water and 0.1% acetic acid, and the mobile phase B was 99.9% methanol and 0.1% acetic acid. The flow rate was 0.8mL / min, the UV detector wavelength is 300nm. The linear gradient analysis program is: 0-30 minutes, 100%A / 0%B to 0%A / 100%B. The rest of the supernatant was adjusted to pH 7.0 with 1N hydrochloric acid and loaded into Amberlite IRC50 (NH 4 + type) resin column, washed with 10 times the column volume of deionized water, and then eluted with 2 liters of 20% ammonium acetate solution. The resulting eluate was mixed with Diaion HP-20 resin and gently shaken at room temperature for 45 minutes for adsorption, then the HP-20 resin...
Embodiment 3
[0077] BLM S (Tianci 102, compound 1) and 6’-dehydroxy-BLM S (Tianci 103, compound 2) DNA cleavage activity test
[0078] in Fe 2+ In the presence of conditions, respectively for BLM S, 6'-dehydroxy-BLM S and bleomycin A 2 (BLMA 2 ) to test and compare their biological activity. Based on the analysis of plasmid relaxation activity of supercoiled plasmid DNA pBluescript II SK(+), single-strand cleavage mediated by bleomycin family compounds first converts supercoiled plasmid DNA (form A) into open circular plasmid DNA (form B ), followed by double-strand cleavage to convert it into linear plasmid DNA (form C). The total reaction volume of DNA cleavage activity test experiment is 10μl, which contains 25mM Tris-HCl buffer (pH 7.5), about 0.65μg of pBluescript SK II (+) plasmid DNA, 5μM Fe(NH 4 ) 2 (SO 4 ) 2 ·6H 2 O (in 1mM H 2 SO 4 freshly prepared in solution) and a certain concentration of active compounds to be tested (BLM S, 6'-dehydroxy-BLM S and BLM A 2 ). After...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com