Preparation method of antioxidant copper nanoparticle
A technology of copper nanoparticles and oxidation resistance, which is applied in the field of transition metal nanoparticle synthesis, can solve problems such as pollution, energy consumption, and long reaction time, and achieve the effects of reducing production costs, strong oxidation resistance, and reduced reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1 Preparation of copper nanoparticles (1)
[0036] Dissolve 6.0 g of anhydrous sodium carbonate in 68 ml of deionized water and stir in a magnetic stirrer. After the anhydrous sodium carbonate is completely dissolved, add 4.30 g of trisodium citrate dihydrate and stir at medium speed for 15 minutes until completely mixed. Get 2 milliliters of 0.68 mol / liter copper sulfate pentahydrate and add dropwise in the above-mentioned mixed solution, the solution color changes from light blue to dark blue, after continuing to stir for 5 minutes, add 3.0 grams of sodium chloride and stir for 15 minutes. After the sodium chloride is completely dissolved, add 1.5 mol / L 30 ml of glucose, stir for 5 minutes, transfer the mixture into a three-necked bottle, seal it, place it in an oil bath at 95°C for 15 minutes, cool it for 24 hours, wash the sample by centrifugation, and dry it. . Place it in a blast oven at 70°C for 24 hours.
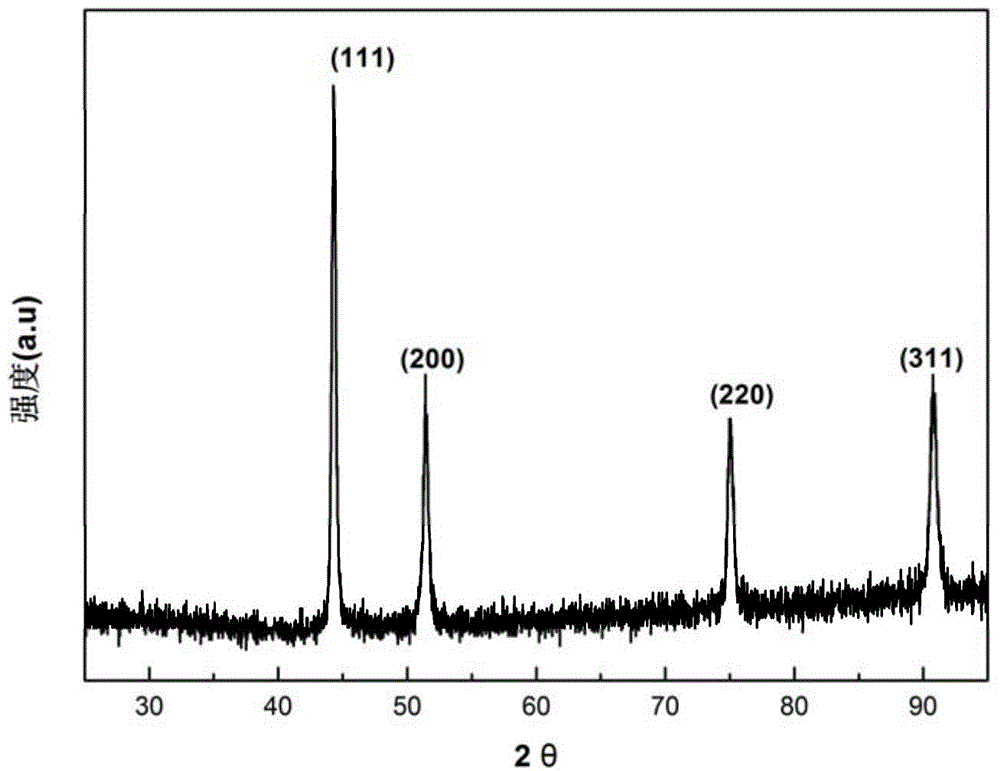
[0037] The X-ray diffraction pattern of the...
Embodiment 2
[0038] Embodiment 2 Preparation of copper nanoparticles (2)
[0039]Dissolve 9.0 g of anhydrous potassium carbonate in 68 ml of deionized water and stir in a magnetic stirrer. After the anhydrous potassium carbonate is completely dissolved, add 4.8 g of potassium citrate monohydrate and stir at a medium speed until completely mixed. Take 4 ml of 0.68 mol / liter copper sulfate pentahydrate and add it dropwise to the above mixed solution. The color of the solution changes from light blue to dark blue. After continuing to stir for 5 minutes, add 1.9 grams of potassium chloride and stir until the potassium chloride is completely dissolved. Add 30 ml of glucose 1.5 mol / L, stir for 5 minutes, transfer the mixture into a three-necked bottle and seal it, place it in a 100°C oil bath and heat for 15 minutes, after cooling, centrifuge to wash the sample, and dry it. Place it in a blast oven at 70°C for 24 hours.
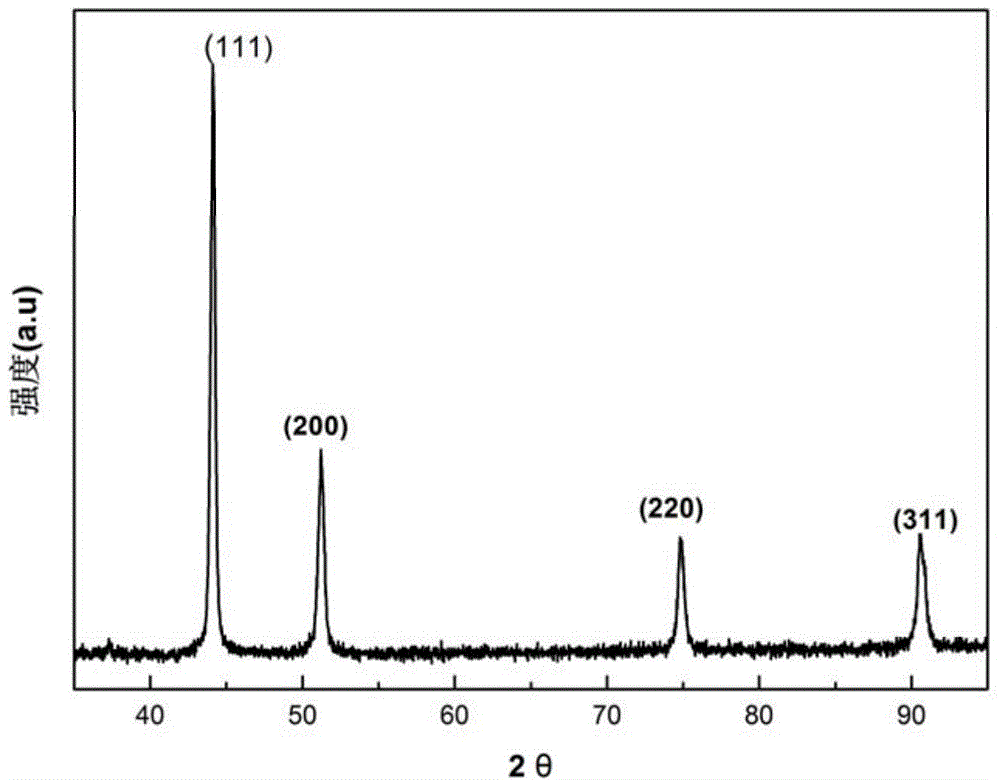
[0040] The X-ray diffraction pattern of the prepared copper nanoparticles...
Embodiment 3
[0041] Embodiment 3 Preparation of copper nanoparticles (3)
[0042] Dissolve 7.0 g of anhydrous sodium carbonate in 68 ml of deionized water and stir in a magnetic stirrer. After the anhydrous sodium carbonate is completely dissolved, add 3.5 g of potassium sodium tartrate and stir until completely mixed. Take 4 ml of 0.68 mol / liter copper sulfate pentahydrate and add it dropwise to the above mixed solution. The color of the solution changes from light blue to dark blue. After stirring for 5 minutes, add 3.0 g of sodium chloride and stir until the sodium chloride is completely dissolved. Add 30 ml of glucose 1.5 mol / L, stir for 5 minutes, transfer the mixture into a three-neck bottle to seal, place in an oil bath at 100°C for 15 minutes, and then centrifuge to wash the sample after cooling, and then dry it. Place it in a blast oven at 70°C for 24 hours.
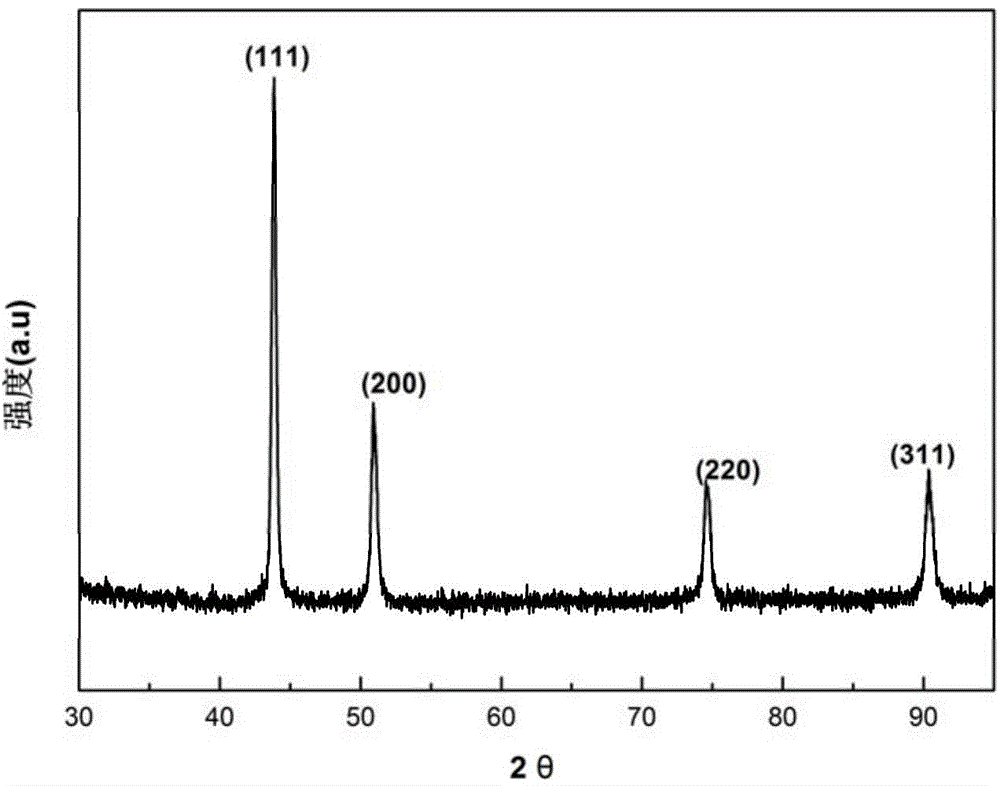
[0043] The X-ray diffraction pattern of the prepared copper nanoparticles is shown in image 3 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com