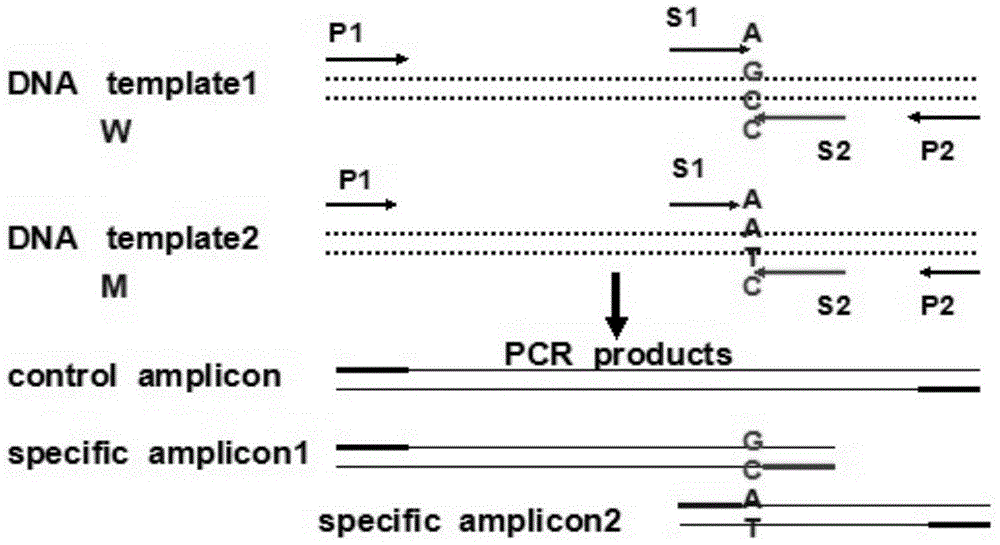
In-vitro diagnostic kit for gene mutation of glucose-6-phosphate dehydrogenase (G6PD) deficiency
A technology of phosphate dehydrogenase and in vitro diagnosis, which is applied in the determination/inspection of microorganisms, biochemical equipment and methods, etc., and can solve the problem of patients not being diagnosed in time or misdiagnosed, unable to distinguish heterozygous or homozygous mutations, and missed detection high rate or false positive rate, achieve high clinical application value, reduce economic and mental burden, and detect quickly and effectively
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
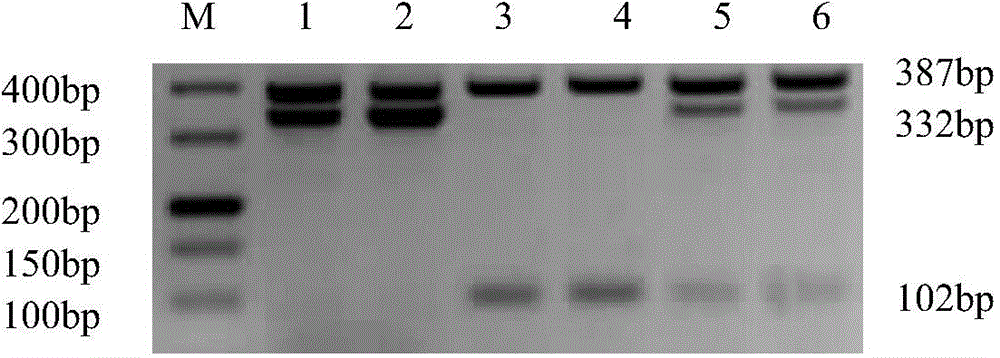
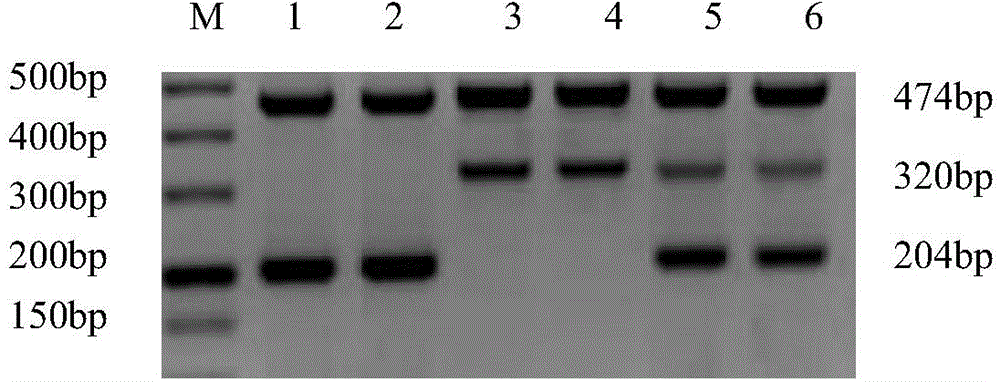
[0046] 1. Sample: DNA extracted from human whole blood (50-100ng / u1). Samples 1 and 2 are normal controls, which means the amplification template is derived from normal human blood DNA without the following 6 high-frequency mutations; samples 3 and 4 are homozygous mutants, which means the amplification template is derived from the following 6 The whole blood DNA of a G6PD-deficient patient with a high-frequency mutation, and the mutation is a homozygous mutation; samples 5 and 6 are heterozygous mutations, which means that the amplification template is derived from a G6PD-deficient patient with the following 6 high-frequency mutations Blood DNA, and the mutation is a heterozygous mutation.
[0047] 2. Reagents: Hot Start Green Master Mix, 2×: Contains 2×Green Go Taq Reaction Buffer (pH8.5), 400μM dATP, 400μM GATP, 400μM dCTP, 400μM dTTP, 4mM MgCl2, Nuclease-Free Water, DNA polymerase.
[0048] 3. T-ARMS-PCR reaction system and amplification program
[0049] ①G1388A uses the follo...
Embodiment 2
[0121] 1. Use the reaction system in Table 1 and the amplification program to perform PCR amplification on each sample. The imaging results of agarose gel electrophoresis are as follows Figure 26 Shown. Sample 1-1 is normal DNA, sample 1-2 is A95G mutant DNA, sample 1-3 is G871A mutant G6PD-deficient patient's whole blood DNA, sample 1-4 is A95G and G871A co-mutated G6PD-deficient patient's whole blood DNA.
[0122] Table 1
[0123]
[0124] PCR amplification program
[0125]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com