Multilayer tablet containing dabigatran etexilate mesylate
A dabigatran etexilate mesylate and methanesulfonic acid-containing technology, applied in the field of multi-layer tablets, to achieve the effects of improving solubility, increasing absorption rate, and increasing bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
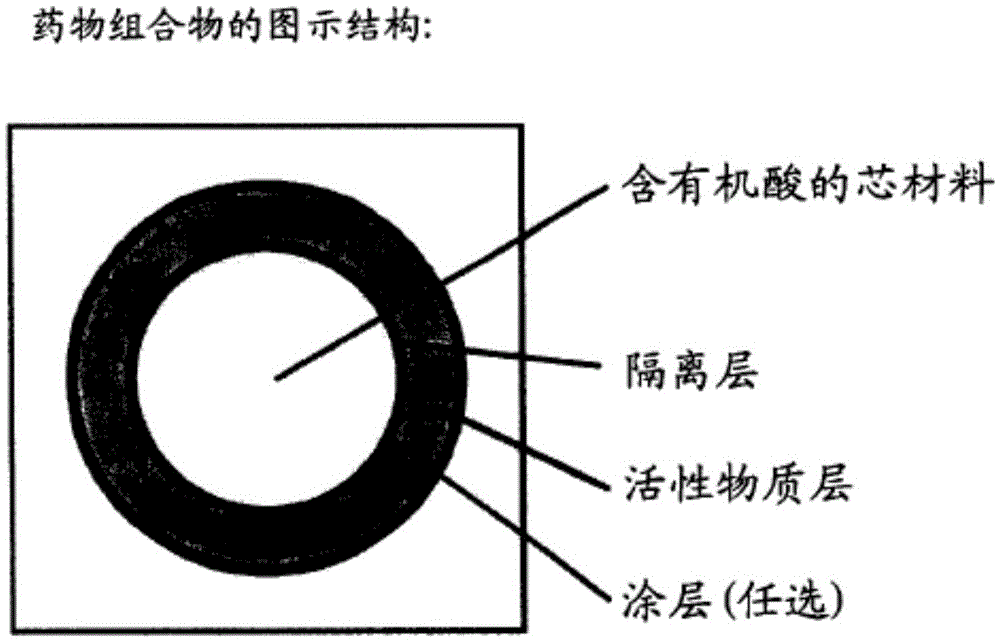
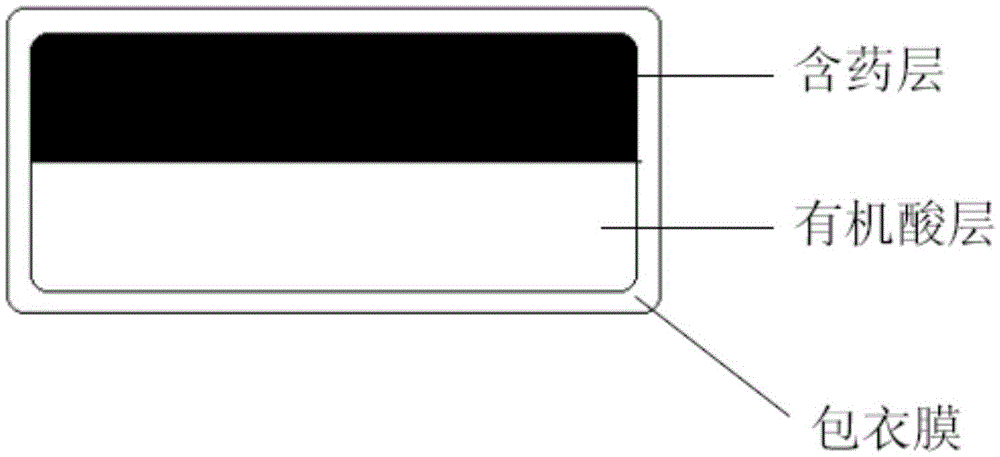
Image
Examples
Embodiment 1
[0022] Example 1: A single-layer tablet containing dabigatran etexilate mesylate, the tablet core prescription is:
[0023]
[0024] In the table: MCC102 is microcrystalline cellulose 102, CC-Na is croscarmellose sodium, HPMCE5 is hypromellose E5, MS is magnesium stearate.
[0025] Single-layer core preparation method:
[0026] (1) Weigh the prescription amount of tartaric acid, dabigatran etexilate mesylate, lactose, MCC102, CC-Na, HPMCE5, add the same amount through a 60 mesh screen and mix three times, add magnesium stearate, and mix by hand for 30S .
[0027] (2) A 14.3x7.5mm shallow arc die is used to press the tablet, and the control pressure is 2000 lbs.
[0028] Coating prescription and process:
[0029] The coating is carried out with a solution of hydroxypropyl cellulose (HPC) in isopropanol with a solid content of 5%. The weight gain of the control coating is 5%. A single-layer coated tablet is obtained.
Embodiment 2
[0030] Example 2: Multi-layer tablets containing dabigatran etexilate mesylate, the tablet core prescription is:
[0031]
[0032] Preparation method of double layer core:
[0033] (1) Preparation of tartaric acid layer
[0034] Weigh the prescription amount of tartaric acid, lactose, MCC102, CC-Na, HPMCE5, pass the same amount through a 60-mesh screen and mix three times, add magnesium stearate, and mix by hand for 30S.
[0035] (2) Preparation of drug-containing layer
[0036] Weigh the prescription amount of dabigatran etexilate mesylate, lactose, MCC102, HPMCE5, CC-Na, and add the same amount through a 60 mesh screen and mix three times, add magnesium stearate, and mix by hand for 30S.
[0037] (3) A 14.3x7.5mm shallow arc punching die is used to press tablets, and the control pressure is 2000 lbs.
[0038] Coating prescription and process:
[0039] The coating is carried out with a solution of hydroxypropyl cellulose (HPC) in isopropanol with a solid content of 5%. The weight gain of...
Embodiment 3
[0041] Example 3: Multi-layer tablets containing dabigatran etexilate mesylate, the tablet core prescription is:
[0042]
[0043]
[0044] Three-layer core preparation method:
[0045] (1) Preparation of tartaric acid layer
[0046] Weigh the prescription amount of tartaric acid, lactose, MCC102, CC-Na, HPMCE5, pass the same amount through a 60-mesh screen and mix three times, add magnesium stearate, and mix by hand for 30S.
[0047] (2) Preparation of drug-containing layer
[0048] Weigh the prescription amount of dabigatran etexilate mesylate, lactose, MCC102, HPMCE5, CC-Na, and add the same amount through a 60-mesh screen and mix three times, add magnesium stearate, and mix by hand for 30S.
[0049] (3) Preparation of isolation layer
[0050] Weigh the same amount of HPMCE5 and MCC102 of the prescription and pass through a 60-mesh screen and mix three times, add magnesium stearate, and mix by hand for 30 minutes.
[0051] (4) A 14.3x7.5mm shallow arc die is used to press the tablet, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com