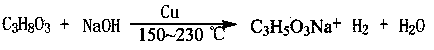Method for preparing lactic acid through catalyzing glycerol by nano copper
A technology for catalyzing glycerin and nano-copper, which is applied in the direction of oxidative preparation of carboxylic acid, carboxylate preparation, carboxylate preparation, etc., can solve the problems of high reaction temperature and reaction pressure, low reaction concentration of glycerin, and long reaction time. Achieve the effects of low reaction temperature and reaction pressure, good industrial prospects, and fast reaction rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Weigh 4.58 g sodium hydroxide (Sinopharm Chemical Reagent Co., Ltd.) and 0.32 g nanometer copper (d 平均 =20 nm), add them together into a 400 mL autoclave, then weigh 9.20 g of glycerol (Sinopharm Chemical Reagent Co., Ltd.) with a 100 mL volumetric flask, add it to the autoclave, and ventilate it with nitrogen , and then airtight, heated to 220 ° C, timed, and reacted for 2 hours.
[0021] At the end of the reaction, take 20 mL of the reaction solution and acidify it with hydrochloric acid until the pH is 2~3, measure the volume after acidification, transfer 1 mL with a pipette and dilute to 25 mL with deionized water, and perform high performance liquid chromatography detection. Measure the production of lactic acid; pipette 1 mL of the acidified sample and 20 μL of n-butanol for gas chromatography analysis; the analysis shows that the conversion rate of glycerol is 100% and the selectivity of lactic acid is 90%.
[0022] Reaction conditions: Glycerol concentration: 1...
Embodiment 2
[0024] Weigh 4.58 g sodium hydroxide (Sinopharm Chemical Reagent Co., Ltd.) and 0.32 g nanometer copper (d 平均 =100 nm), add them together into a 400 mL autoclave, then weigh 9.20 g of glycerol (Sinopharm Chemical Reagent Co., Ltd.) with a 100 mL volumetric flask to constant volume, add it to the autoclave, and ventilate it with nitrogen , and then airtight, heated to 230 ° C, timed, and reacted for 0.5 hours.
[0025] At the end of the reaction, take 20 mL of the reaction solution and acidify it with hydrochloric acid until the pH is 2~3, measure the volume after acidification, transfer 1 mL with a pipette and dilute to 25 mL with deionized water, and perform high performance liquid chromatography detection. The production of lactic acid was measured; then 20 μL of 1 mL acidified sample was pipetted for gas chromatography analysis; the analysis showed that the conversion rate of glycerol was 93% and the selectivity of lactic acid was 88%.
[0026] Reaction conditions: Glycero...
Embodiment 3
[0028] Weigh 4.58 g sodium hydroxide (Sinopharm Chemical Reagent Co., Ltd.) and 0.32 g nanometer copper (d 平均 =150 nm), add them together into a 400 mL autoclave, weigh 9.20 g of glycerin (Sinopharm Chemical Reagent Co., Ltd.) with a 100 mL volumetric flask, add it to the autoclave, and ventilate it with nitrogen , and then airtight, heated to 220 ° C, timed, and reacted for 2 hours.
[0029] At the end of the reaction, take 20 mL of the reaction solution and acidify it with hydrochloric acid until the pH is 2~3, measure the volume after acidification, transfer 1 mL with a pipette and dilute to 25 mL with deionized water, and perform high performance liquid chromatography detection. The production of lactic acid was measured; then 20 μL of 1 mL acidified sample was pipetted and analyzed by gas chromatography; the analysis showed that the conversion rate of glycerol was 83%, and the selectivity of lactic acid was 82%.
[0030] Reaction conditions: Glycerol concentration: 1.0 M...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com