Sialidase with metal ion tolerance, heat stability and acid stability and application thereof
A technology of sialidase and thermal stability, applied in the field of sialidase, can solve the problems of difficult to obtain, weak substrate specificity, complicated enzyme purification process, etc., and achieves inhibition of migration and strong tolerance to divalent metal ions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: the acquisition of sialidase
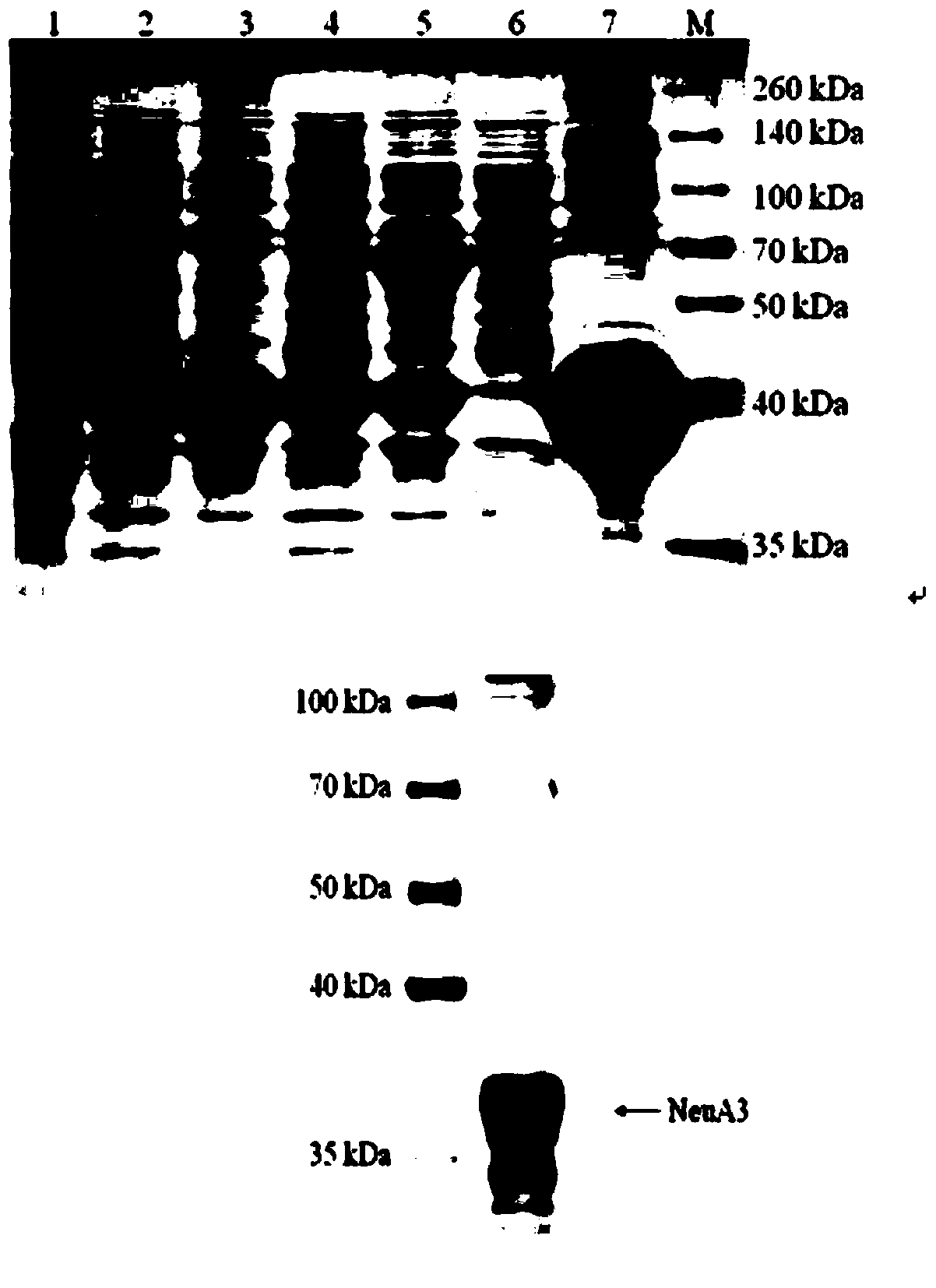
[0030] 1. Cloning and expression
[0031] The sequence information of the neuA3 gene was obtained through the website http: / / avermitilis.ls.kitasato-u.ac.jp / , and the signal peptide of the enzyme was predicted through the SignalP4.1Server website, and the results showed that the enzyme did not contain a signal peptide A small sialidase of approximately 38 kDa in size. Primers were designed in the coding region of the neuA3 gene, and the following primers were synthesized by the company: neuA3-S: GGAATTCCATATGGCGATGACAGAAGCCAGTACAC (the restriction site of EcoRI is underlined) and neuA3-AS: CCCAAGCTTCAGTAGAGGTCATGGGGTGA (the restriction site of HindIII is underlined), Using the Streptomyces avermitilis genome extracted above as a template for PCR amplification, the amplification conditions were as follows: 95°C for 10 min, 96°C for 1 min, 65°C for 45 s, 72°C for 1 min and 30 s for 34 cycles, and 72°C for 10 min. The PCR ampl...
Embodiment 2
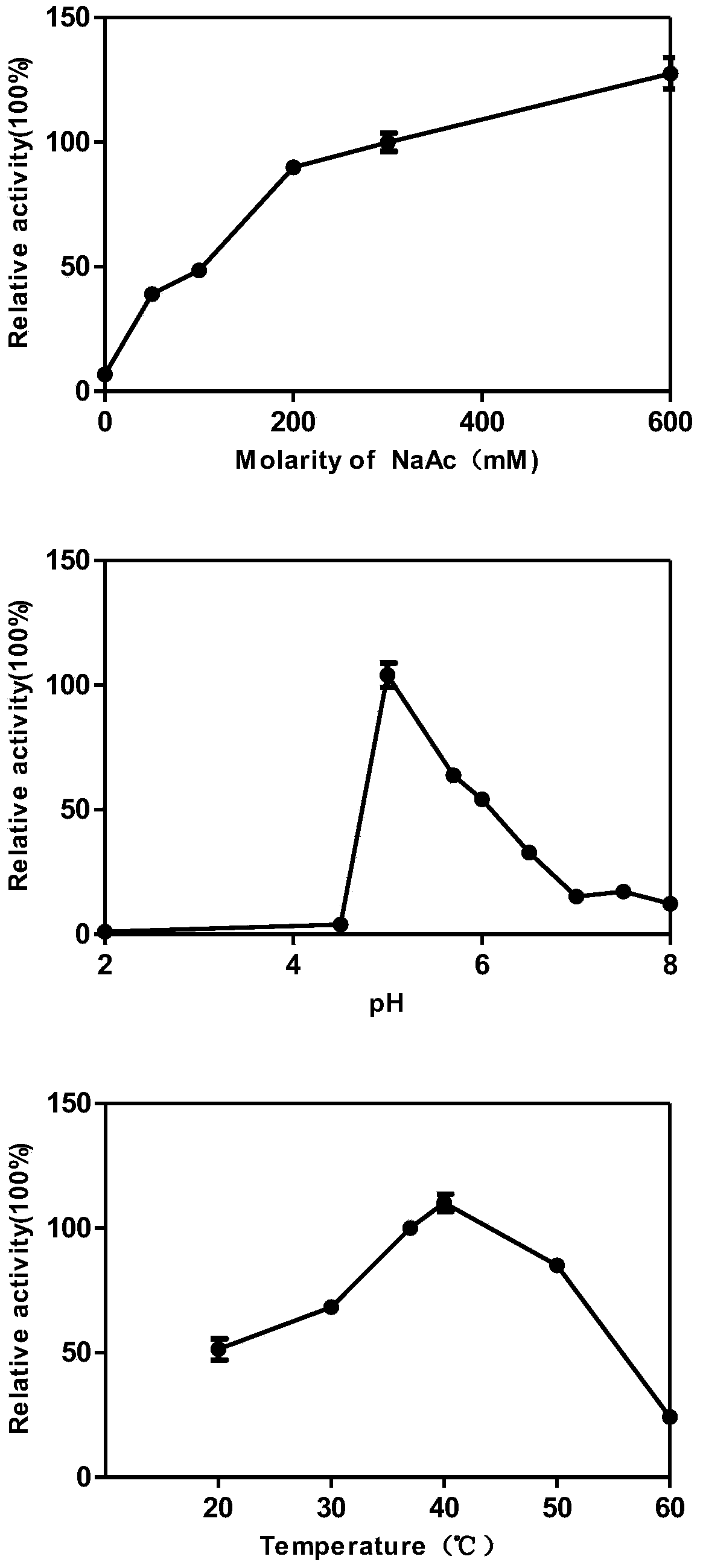
[0042] The enzymatic property research of embodiment 2 novel sialidases
[0043]Dilute the enzyme solution to 0.1 mg / mL, use the derivative of Neu5Ac 4-MU-Neu5Ac as the substrate, add 10 μL of the enzyme solution of this concentration, 10 μL of 1 mM substrate 4-MU-Neu5Ac, and 80 μL of reaction buffer After reacting for a certain period of time, add 100 μL of stop buffer (glycine 0.133M, NaCl 0.06M, NaCl 2 CO 3 0.083M, pH10.7) to terminate the enzymatic hydrolysis reaction to obtain free 4-MU, because free 4-MU can produce 450nm fluorescence at an excitation wavelength of 365nm, while non-free 4-MU-Neu5Ac can only produce 450nm fluorescence at this excitation wavelength A weak fluorescent signal is generated, so the fluorescent signal of free 4-MU can be collected by a fluorescent microplate reader, and the enzyme activity can be calculated according to the standard curve of the relationship between the fluorescent signal and the concentration of 4-MU. One enzyme activity uni...
Embodiment 3
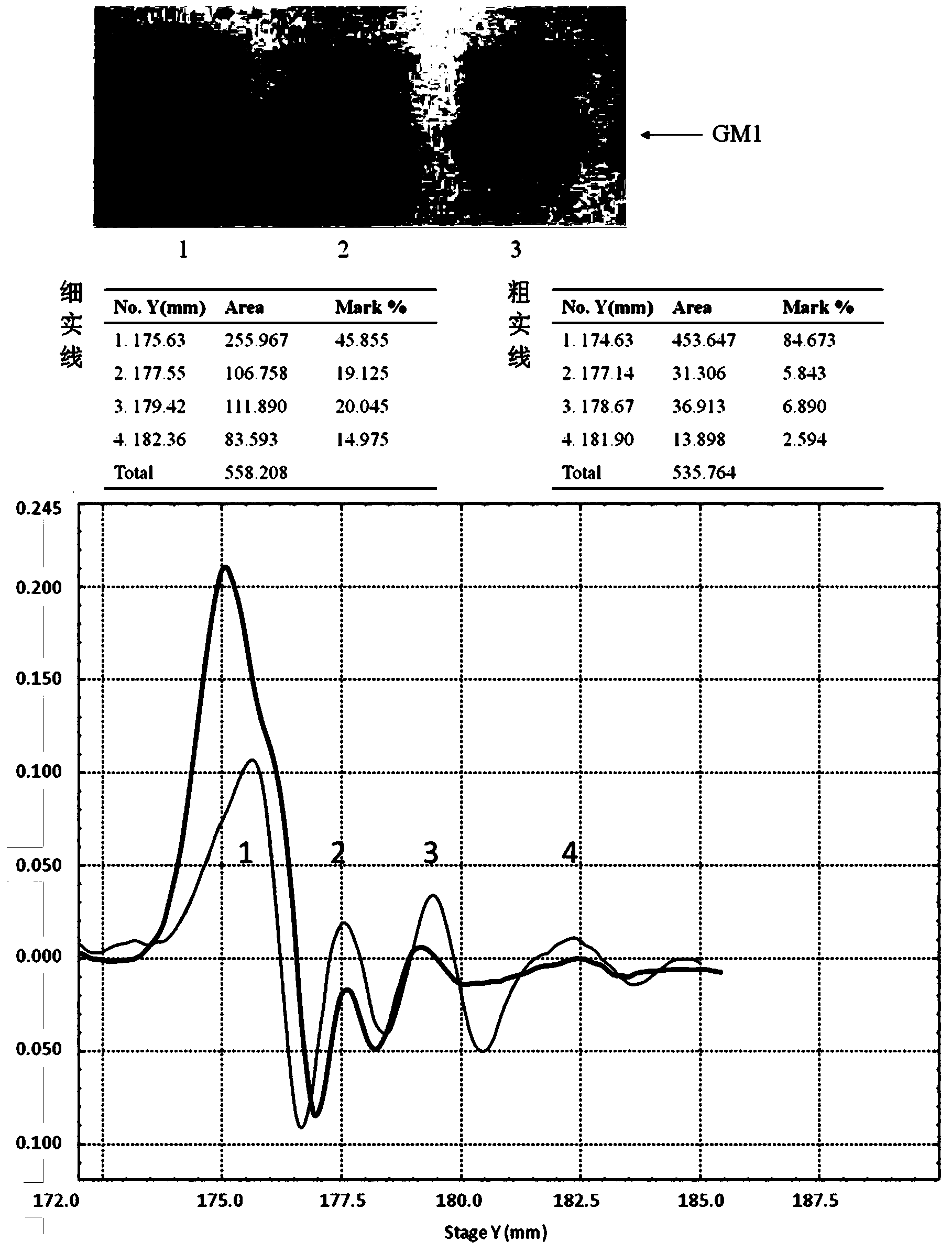
[0048] Example 3 Application research of sialidase biotransformation of gangliosides
[0049] Mix a certain concentration of enzyme and total gangliosides extracted from pig brain in the reaction buffer, and after the reaction is terminated, dry the reacted system with a nitrogen blower and then use chloroform / methanol (V / V =2 / 1) solution was redissolved, the total gangliosides before the reaction was used as a control, and the ganglioside standard was used as a Marker to carry out HPTLC, and the silica gel plate after the expansion layer was used for color reaction with orcinol. At the same time, the silica gel plate after spreading can also be used for immunoblotting experiments. Soak the silica gel plate in 1% BSA / PBS (W / V), seal at room temperature for 30 minutes, and add biotin markers specifically recognized by corresponding gangliosides in a certain proportion. After incubation at room temperature for 2 hours, wash with TBS-T three times, each time for 5 minutes. Add h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com