Resist underlayer film forming composition containing copolymer resin having heterocyclic ring
一种抗蚀剂下层、组合物的技术,应用在含有具有杂环的共聚树脂的抗蚀剂下层膜形成用组合物领域,能够解决难以获得抗蚀剂图案膜厚等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
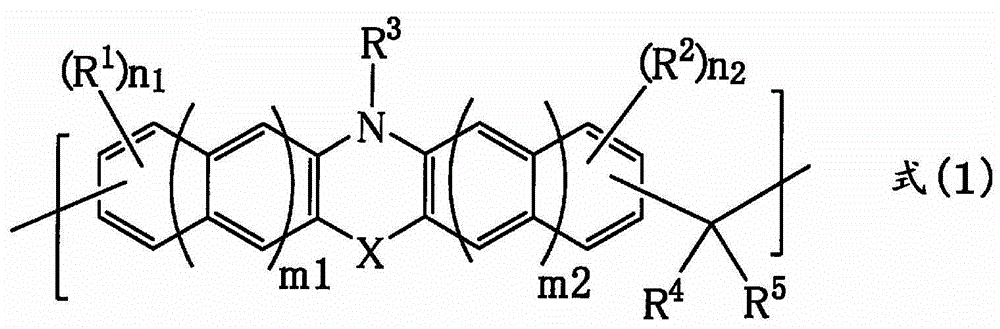
[0170] 8.0 g of phenothiazine (manufactured by Tokyo Chemical Industry Co., Ltd.), 6.3 g of 1-naphthaldehyde (manufactured by Tokyo Chemical Industry Co., Ltd.), p-toluenesulfonic acid monohydrate (manufactured by Tokyo Chemical Industry Co., Ltd.) were added to a 100 ml pear-shaped bottle. system) 0.81g, 1,4-di Alkane (manufactured by Kanto Chemical Industry Co., Ltd.) 35.2 g. Then, the inside of the flask was replaced with nitrogen, and then heated and stirred under reflux for about 28 hours. After the reaction, use 1,4-di Diluted with 10 g of alkane (manufactured by Kanto Chemical Industry Co., Ltd.). The diluted solution was added dropwise to a mixed solution of methanol / 28% ammonia water (500 g / 5 g) to cause reprecipitation. The obtained precipitate was suction-filtered, the filter residue was washed with methanol, and then dried under reduced pressure at 85° C. overnight to obtain 5.8 g of novolak resin. The obtained polymer corresponds to formula (2-1). The weigh...
Synthetic example 2
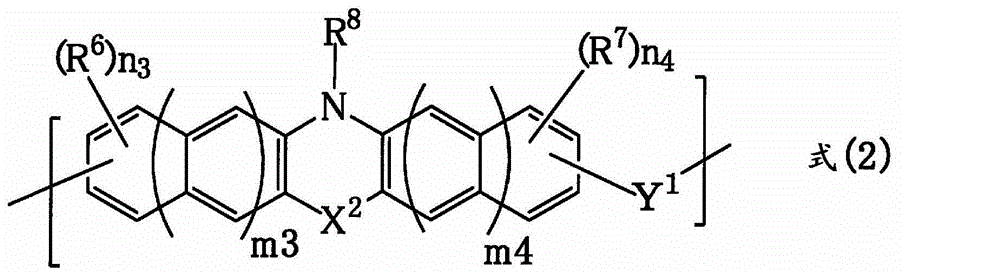
[0172] 12.0 g of phenothiazine (manufactured by Tokyo Chemical Industry Co., Ltd.), 6.4 g of benzaldehyde (manufactured by Tokyo Chemical Industry Co., Ltd.), and p-toluenesulfonic acid monohydrate (manufactured by Tokyo Chemical Industry Co., Ltd.) were added to a 100 ml pear-shaped bottle 1.2g, 1,4-di Alkanes (manufactured by Kanto Chemical Industry Co., Ltd.) 112.8 g. Then, the inside of the flask was replaced with nitrogen, and then heated and stirred under reflux for about 26 hours. After completion of the reaction, it was diluted with 22 g of tetrahydrofuran (manufactured by Kanto Chemical). The diluted solution was added dropwise to 750 g of methanol to cause reprecipitation. The obtained precipitate was suction-filtered, the filter residue was washed with methanol, and then dried under reduced pressure at 85° C. overnight to obtain 2.2 g of novolak resin. The obtained polymer corresponds to formula (2-2). The weight average molecular weight Mw measured by polystyr...
Synthetic example 3
[0174] 10.0 g of phenothiazine (manufactured by Tokyo Chemical Industry Co., Ltd.), 9.1 g of 9-fluorenone (manufactured by Tokyo Chemical Industry Co., Ltd.), and p-toluenesulfonic acid monohydrate (manufactured by Tokyo Chemical Industry Co., Ltd.) were added to a 100 ml pear-shaped bottle. manufactured) 1.0 g, toluene (manufactured by Kanto Chemical Industry) 46.8 g. Then, the inside of the flask was replaced with nitrogen, and then heated and stirred under reflux for about 29 hours. After completion of the reaction, it was diluted with 10.4 g of tetrahydrofuran (manufactured by Kanto Chemical). The diluted solution was added dropwise to 800 ml of methanol for reprecipitation. The obtained precipitate was suction-filtered, the filter residue was washed with methanol, and then dried under reduced pressure at 85° C. overnight to obtain 12.4 g of novolac resin. The obtained polymer corresponds to formula (2-3). The weight average molecular weight Mw measured by polystyrene c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight-average molecular weight | aaaaa | aaaaa |
| weight-average molecular weight | aaaaa | aaaaa |
| weight-average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com