Fusion protein containing leucine-rich repetitive sequence, and preparation method and application thereof
A technology of fusion protein and protein, applied in the field of fusion protein, can solve the problems of many impurities, inaccurate expression of target protein, cumbersome process and steps, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
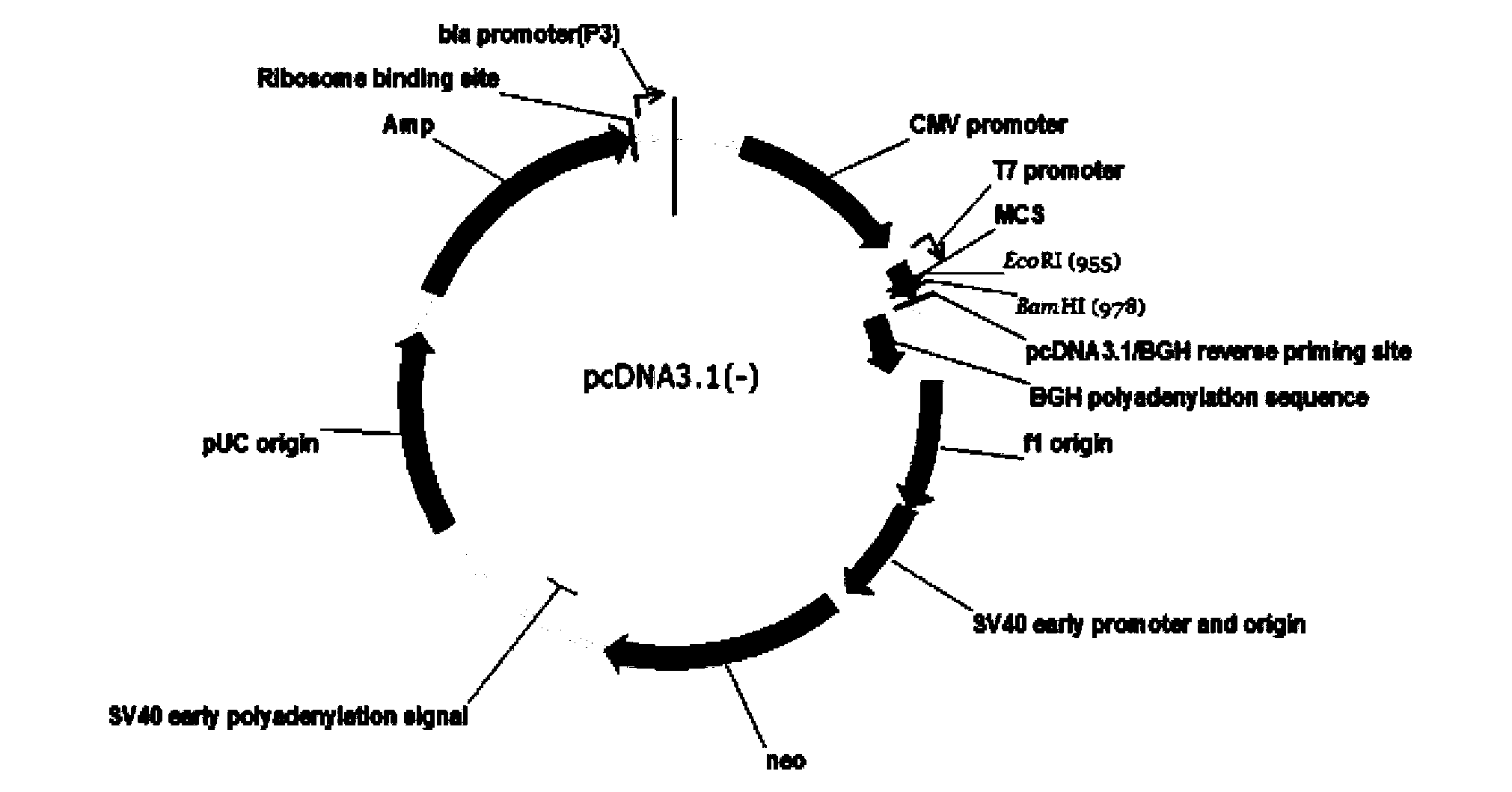
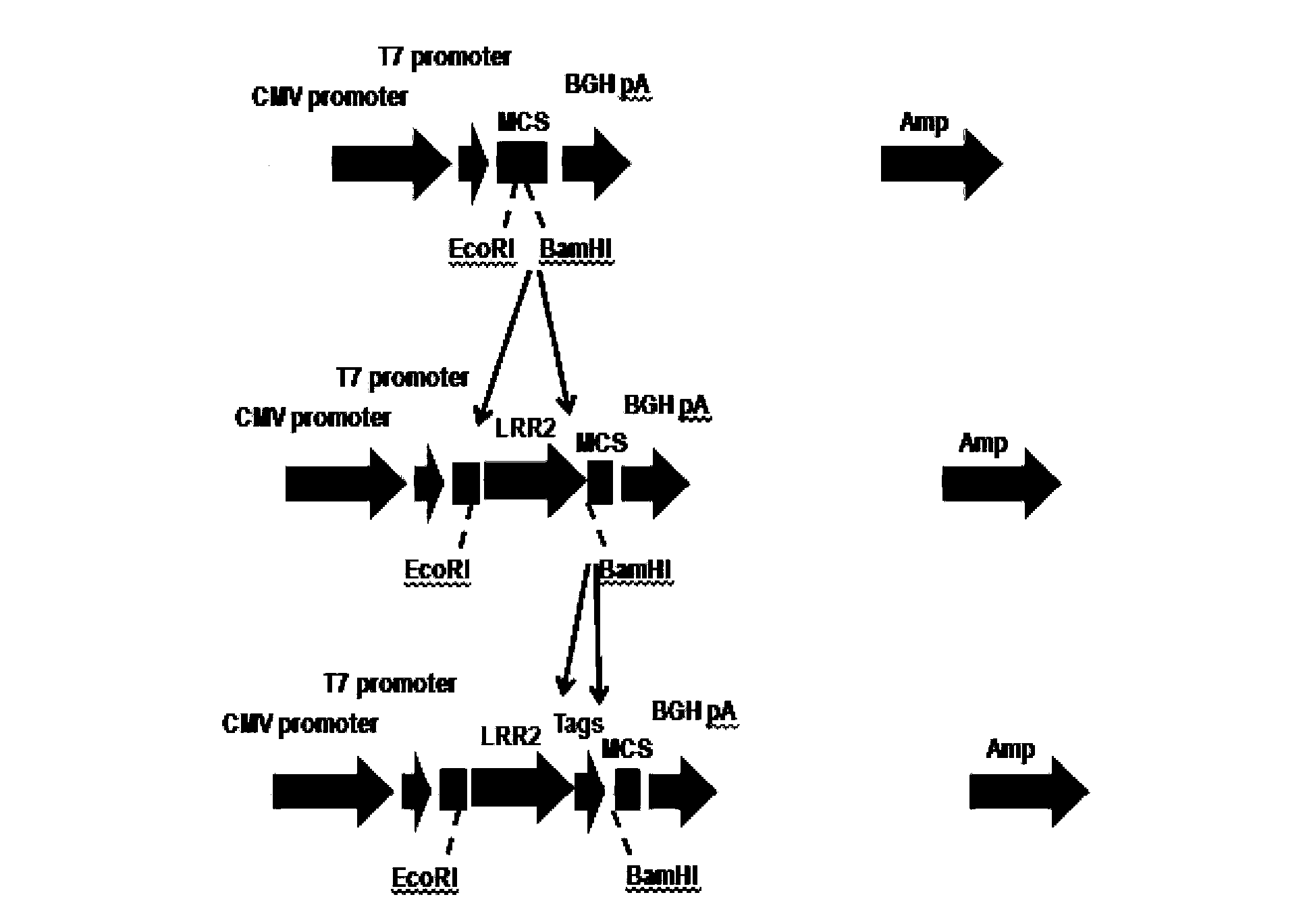
[0128] Example 1 Construction of pcDNA3.1(-) / Slit3LRR2 vector
[0129] Method: The plasmid was constructed by exonuclease method. In the process of constructing the plasmid, a total of three fragments (signal peptide sequence, target sequence, restriction site + tag protein sequence) were amplified by PCR, and the fragments were ligated and inserted three times. The primers used were Primer1, Primer2 and Primer3, respectively.
[0130] Specific steps of exonuclease method:
[0131] 1. Prepare the reaction system: 50ng each of the carrier fragment (recovered from the gel after digestion with BamHI and measure its concentration) and PCR fragment (recovered from the gel after PCR and measure its concentration), 1ul10x exonuclease buffer, supplemented with ddH 2 0 to 10ul;
[0132] 2. Place on ice for 5 minutes, add 1 ul exonuclease III (Takara company) diluted to 20 U / ul, mix well and place on ice for 60 minutes;
[0133] 3. Add 1ul 0.5M EDTA (pH8.0) solution to terminate the r...
Embodiment 2
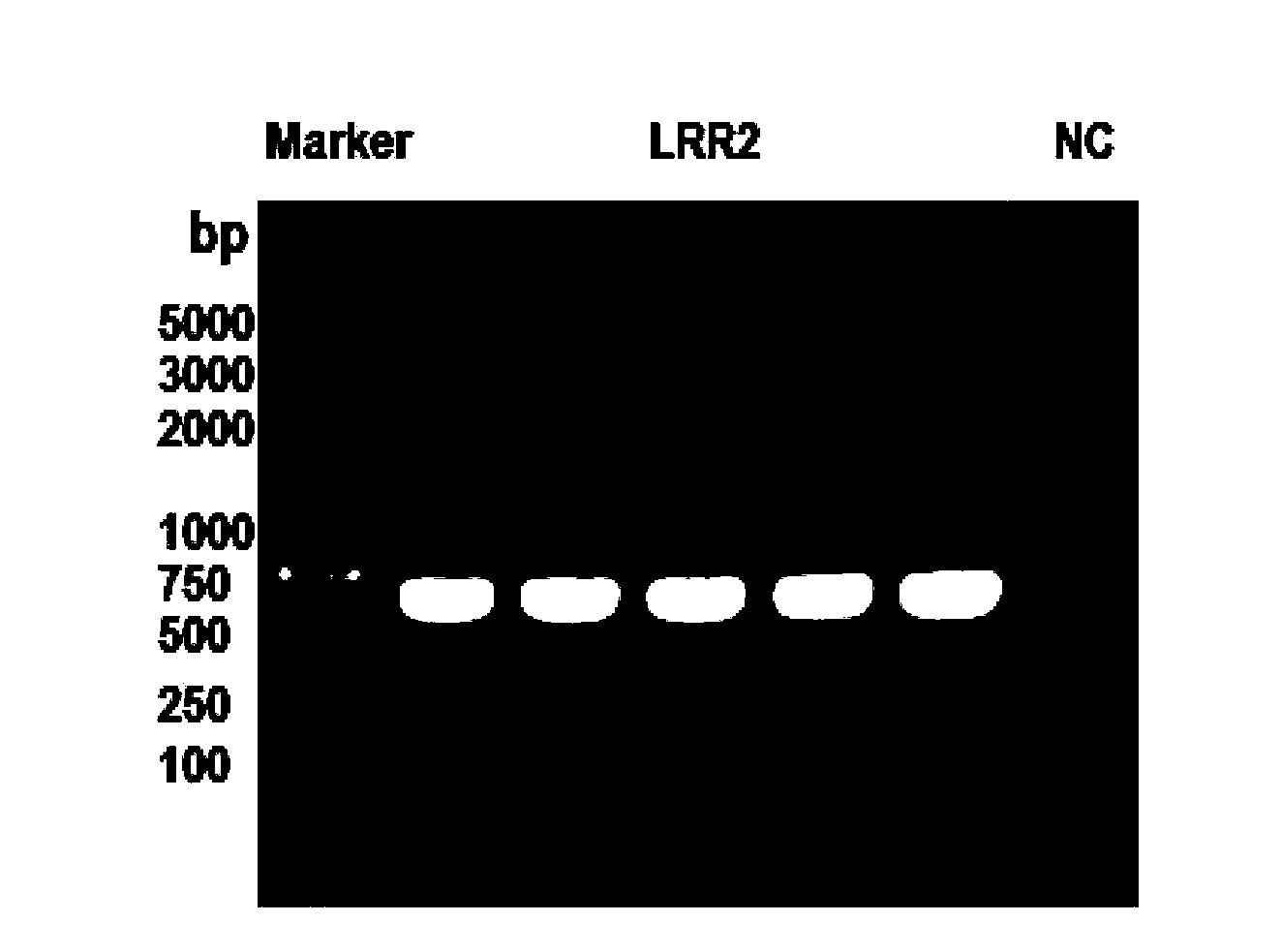
[0144] Example 2 Successful Expression of pcDNA3.1(-) / Slit3LRR2
[0145] Method: In order to detect whether the constructed plasmid was successfully expressed, the plasmid was transfected into AD293 cells, and then Western Blot was done with the tag protein 8×His to detect whether the fusion protein was successfully expressed.
[0146] Materials: High glucose DMEM medium and fetal bovine serum were purchased from HyClone Company; transfection reagent Magetran was purchased from Origene Company; AD293 cells were cultured in High glucose DMEM medium containing 10% fetal bovine serum. The inverted microscope is a product of Olympus, and the fluorescent inverted microscope is a product of Nikon.
[0147] step:
[0148] 2.1. Resuscitate and culture the cryopreserved cells, passage 3 to 4 times to make the cells reach a good growth state, and carry out plate detection. Adherent cells were transfected with Magetran reagent.
[0149] 2.2. Inoculate AD293 cells into a 6-well plate a...
Embodiment 3
[0152] The test of embodiment 3 expression amount
[0153] A purified HisGFP protein with a known concentration (20 μg / ml) was used as a control, and the protein expression was determined by Western Blot.
[0154] The specific method is the same as that in Example 2, the cells are first transfected, and then Western Blot detection is performed. The difference is that when running SDS-PAGE electrophoresis, a certain known amount of HisGFP protein is added as a positive control.
[0155] After the exposure results were obtained, the expression level of the protein was calculated by performing grayscale analysis on the bands.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com