Praseodymium holmium co-doped niobate up-conversion luminescent material, preparation method and organic light-emitting diodes
A luminescent material, co-doping technology, applied in luminescent materials, chemical instruments and methods, semiconductor/solid-state device manufacturing, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] The preparation method of the above-mentioned praseodymium-holmium co-doped niobate up-conversion luminescent material comprises the following steps:
[0029] Step S11, according to MeNbO 3 :xPr 3+ ,yHo 3+ The stoichiometric ratio of each element is called Me 2 O, Nb 2 o 5 , Pr 2 o 3 and Ho 2 o 3 Powder, wherein x is 0.01-0.08, y is 0.01-0.06, and Me is one of lithium, sodium, potassium, rubidium and cesium.
[0030] In this step, preferably, x is 0.04 and y is 0.03.
[0031] Step S13, adding an acidic solvent to the powder weighed in step S11, and then simultaneously adding a dispersant and an alkaline solvent to obtain a mixture containing precipitates.
[0032] In this step, preferably, the acidic solvent is hydrofluoric acid, ammonium fluoride or ammonium bifluoride.
[0033] In this step, preferably, the dispersant is oxalic acid, ethanol, triethanolamine, water-soluble starch or polyethylene glycol.
[0034] In this step, preferably, the alkaline solve...
Embodiment 1
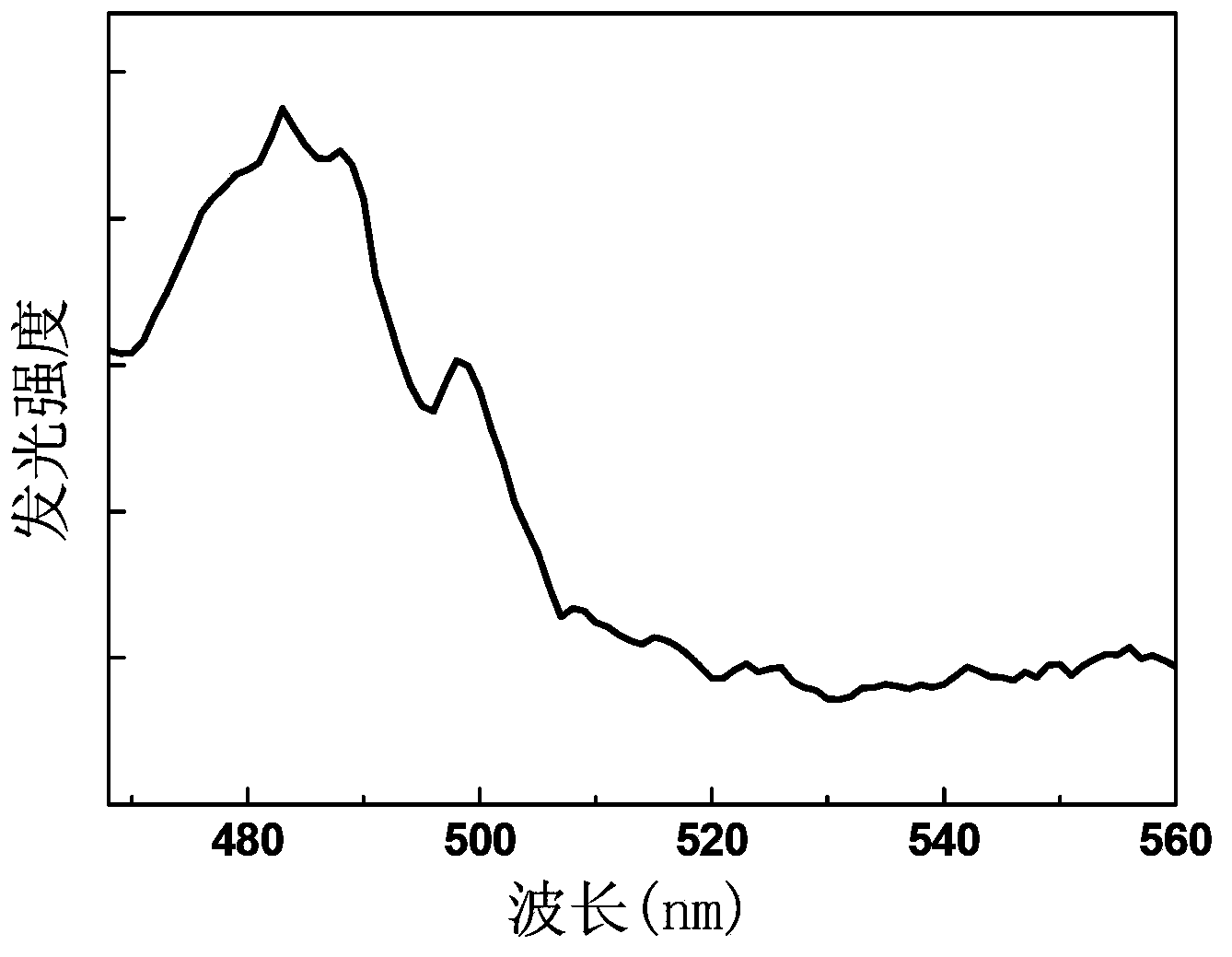
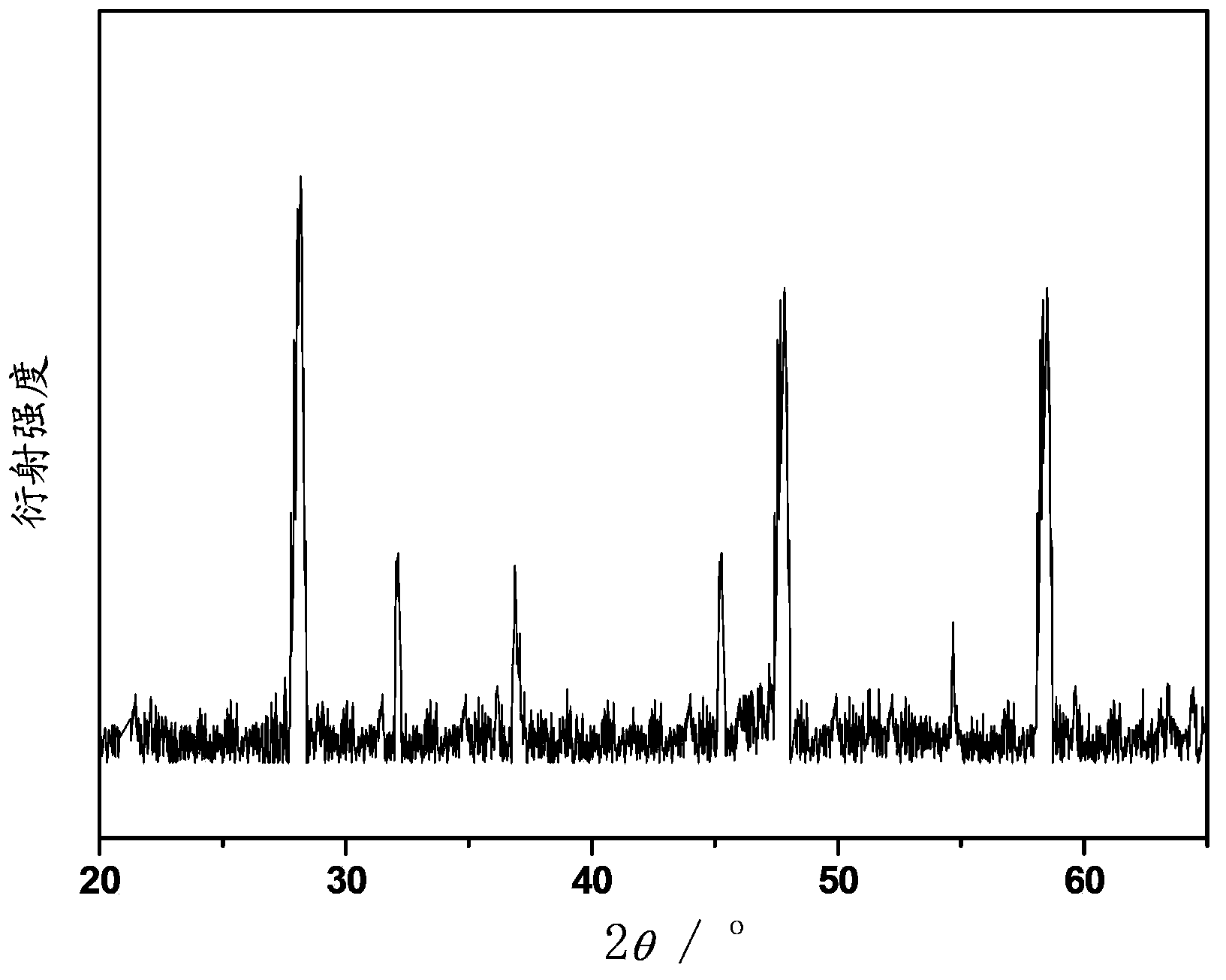
[0045] Choose Li 2 O, Nb 2 o 5 , Pr 2 o 3 and Ho 2 o 3 The powder is mixed according to the molar number of each component being 1mmol, 1mmol, 0.04mmol and 0.03mmol. After mixing, dissolve in the hydrochloric acid solvent, add oxalic acid dropwise as a dispersant and add ammonia water to make the mixed solution no longer precipitate, continue to add ammonia water dropwise, adjust the pH value of the mixed solution to about 8, let it stand for 2 hours to make the precipitation complete, and use a filter bucket Collect the precipitate by filtration, then wash the precipitate repeatedly with absolute ethanol and distilled water, and finally place the collected precipitate in a muffle furnace and bake at 1000°C for 2 hours to obtain the general chemical formula of LiNbO 3 : 0.04Pr 3+ , 0.03Ho 3+ Praseodymium holmium co-doped niobate up-conversion luminescent material.
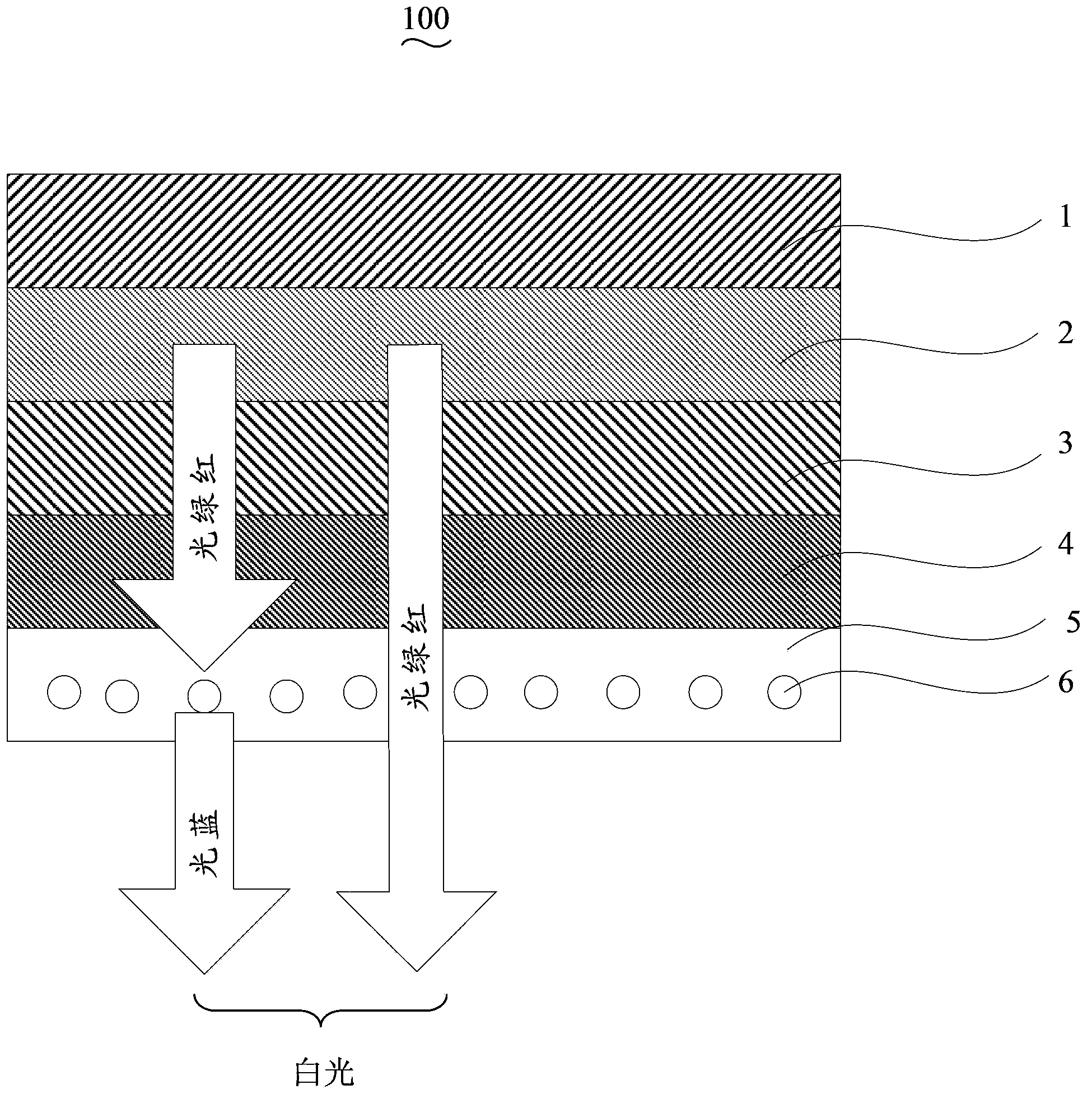
[0046] The sequentially stacked substrate 1 uses soda-lime glass, the cathode 2 uses a metal Ag layer, ...
Embodiment 2
[0051] Choose Li 2 O, Nb 2 o 5 , Pr 2 o 3 and Ho 2 o 3 The powder is mixed according to the molar number of each component being 1mmol, 1mmol, 0.01mmol and 0.01mmol. After mixing, dissolve in the hydrochloric acid solvent, add ethanol dropwise as a dispersant, and add ammonia water at the same time so that the mixed solution no longer produces precipitation, continue to add ammonia water dropwise, adjust the pH value of the mixed solution to about 8, let it stand for 2 hours to make the precipitation complete, and use a filter bucket Collect the precipitate by filtration, then wash the precipitate repeatedly with absolute ethanol and distilled water, and finally place the collected precipitate in a muffle furnace and bake at 900°C for 2 hours to obtain the general chemical formula LiNbO 3 : 0.01Pr 3+ , 0.01Ho 3+ Praseodymium holmium co-doped niobate up-conversion luminescent material.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com