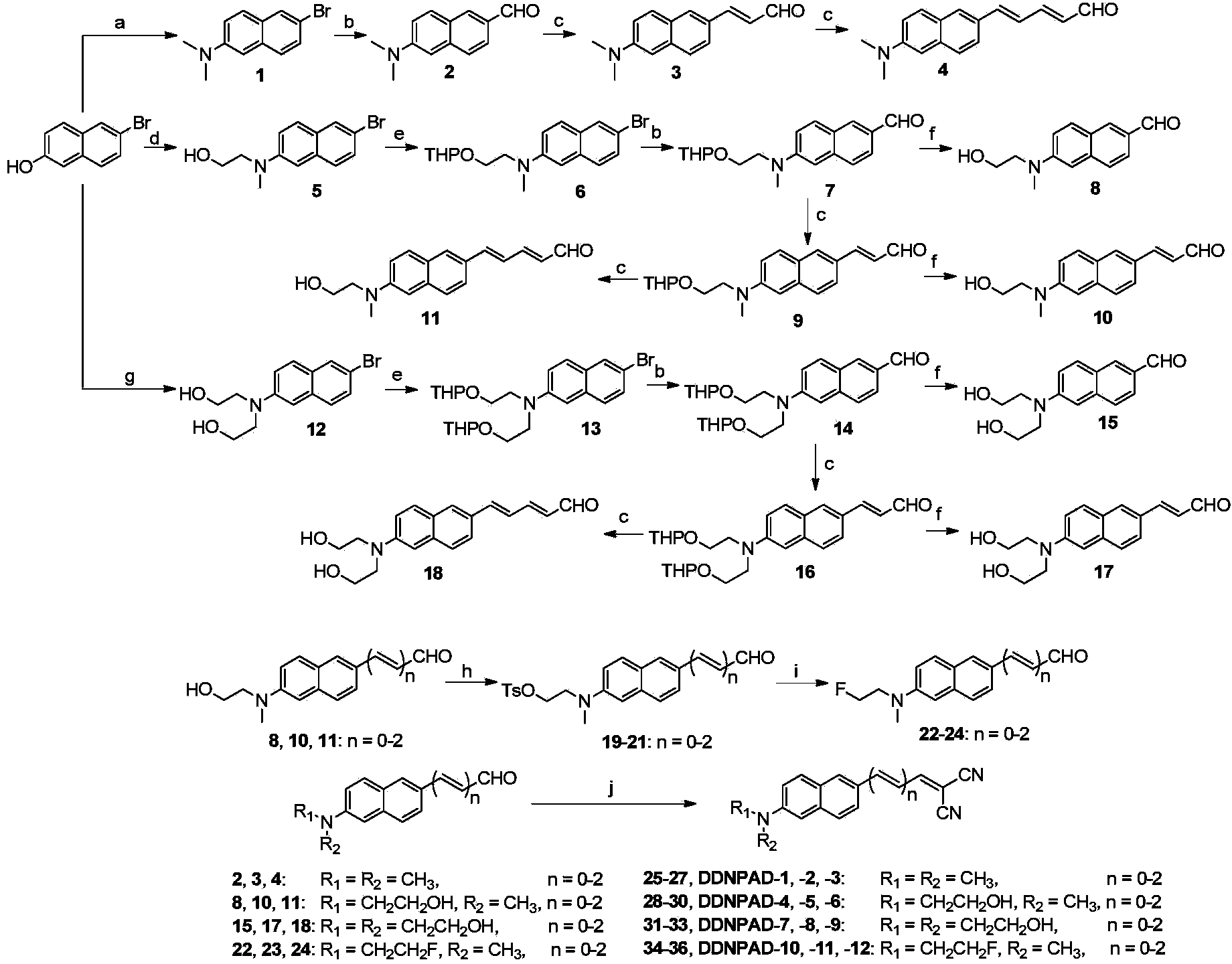Compound provided with good affinity and tangled with Abeta plaque and nerve fiber and preparation method thereof
A neurofibrillary tangles and compound technology, which is applied in the preparation of organic compounds, cyanide reaction preparation, chemical instruments and methods, etc., can solve the problems of large molecular weight, weak Aβ plaque affinity, and complex compound synthesis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1 Synthesis of derivatives based on naphthalene ring
[0055] 1. Synthetic intermediate 1
[0056] After 5.5 g of 6-bromo-2-naphthol was dissolved in 35 mL of dimethylamine aqueous solution (33%), 8.2 g of Na 2 S 2 o 5 , reacted in a 140°C autoclave for 18 hours. After stopping the reaction, ultrasonically wash with NaOH aqueous solution (1M) for 15 minutes, filter with suction, then wash the solid with NaOH aqueous solution (1M) and deionized water respectively, and dry at 70°C to obtain 4.4 g of light green solid. The yield is 65.8%, and the structure is as follows: 1 H NMR (400MHz, CDCl 3 )δ:7.83(s,1H),7.62(d,J=9.0Hz,1H),7.53(d,J=8.7Hz,1H),7.42(d,J=8.8Hz,1H,7.19(d,J =8.0Hz, 1H), 6.92(s, 1H), 3.06(s, 6H).
[0057] 2. Synthetic intermediate 2
[0058] After dissolving 11.4g of intermediate 1 in 50mL of anhydrous tetrahydrofuran, add 27.5mL of n-butyllithium (2.5M n-hexane solution) under nitrogen protection at -78°C, and stir for 30 minutes; then add 5....
Embodiment 2
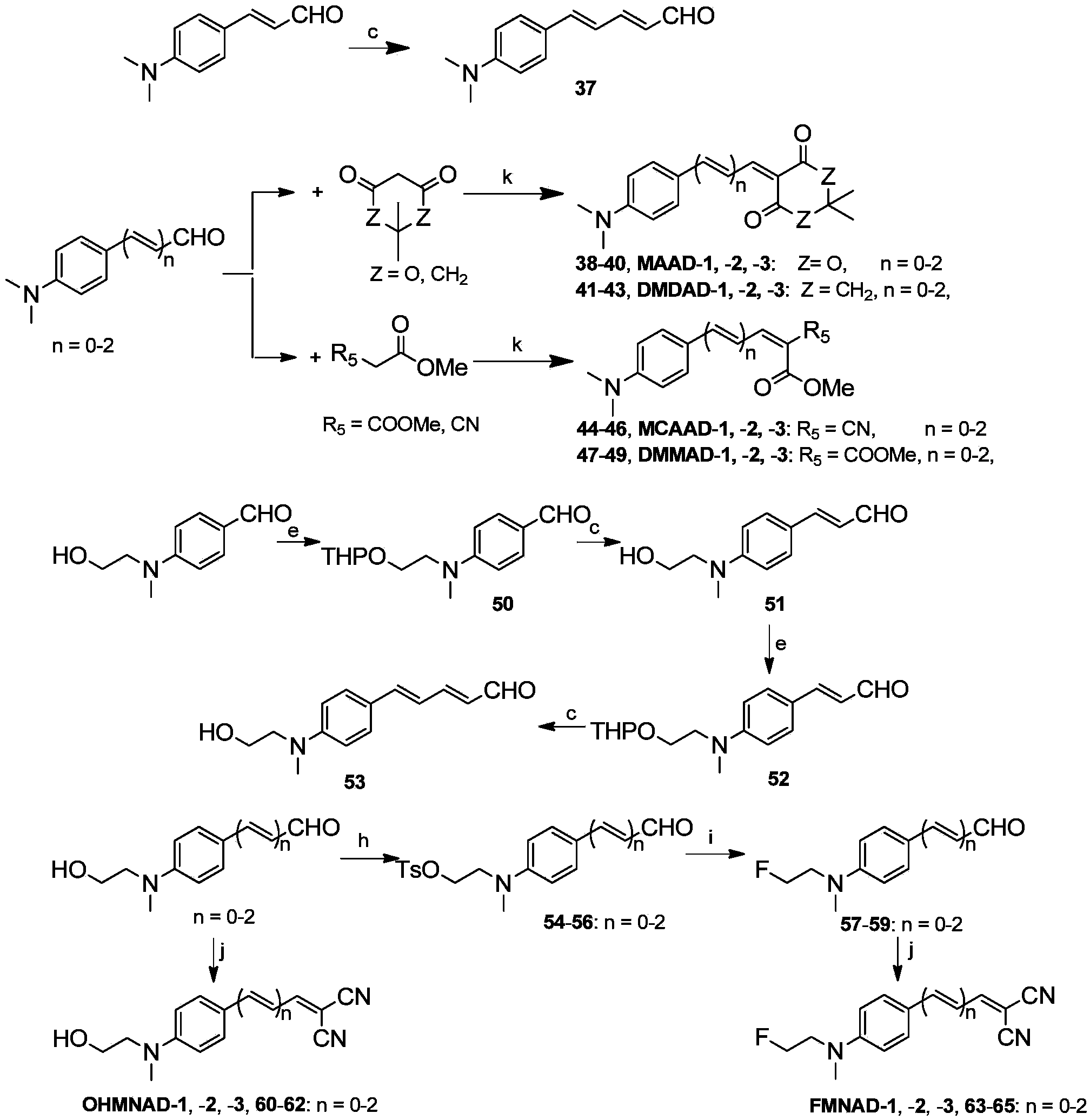
[0128] Example 2 Synthesis of Molecular Probes Based on Benzene Rings
[0129] 37. Synthetic intermediate 37
[0130] 4-Dimethylaminocinnamaldehyde (578mg, 3.3mmol) and (1,3-dioxolanyl-2-methyl)triphenylphosphine bromide (1.36g, 3.2mmol) were dissolved in 25mL of anhydrous THF , 70 ° C reflux reaction 2h. Concentrated hydrochloric acid was added to the reaction system, and then the pH was adjusted to 7 with ammonia water. CH 2 Cl 2 The aqueous phase was extracted three times, the organic phase was dried over anhydrous magnesium sulfate, the solvent was removed by rotary evaporation, and column separation (petroleum ether: ethyl acetate = 4:1, volume ratio) gave a yellow solid (139 mg, 46.0%). 1 H NMR (400MHz, CDCl 3 )δ: 9.56(d, J=8.1Hz, 1H), 7.41(d, J=8.9Hz, 2H), 7.32-7.21(m, 1H), 6.95(d, J=15.3Hz, 1H), 6.82( dd, J = 15.3, 10.9 Hz, 1H), 6.68 (d, J = 8.8 Hz, 2H), 6.18 (dd, J = 15.0, 8.1 Hz, 1H), 3.03 (s, 6H).
[0131] 38. Synthesis of compound 38 (MAAD-1)
[0132] 4-Dim...
Embodiment 3
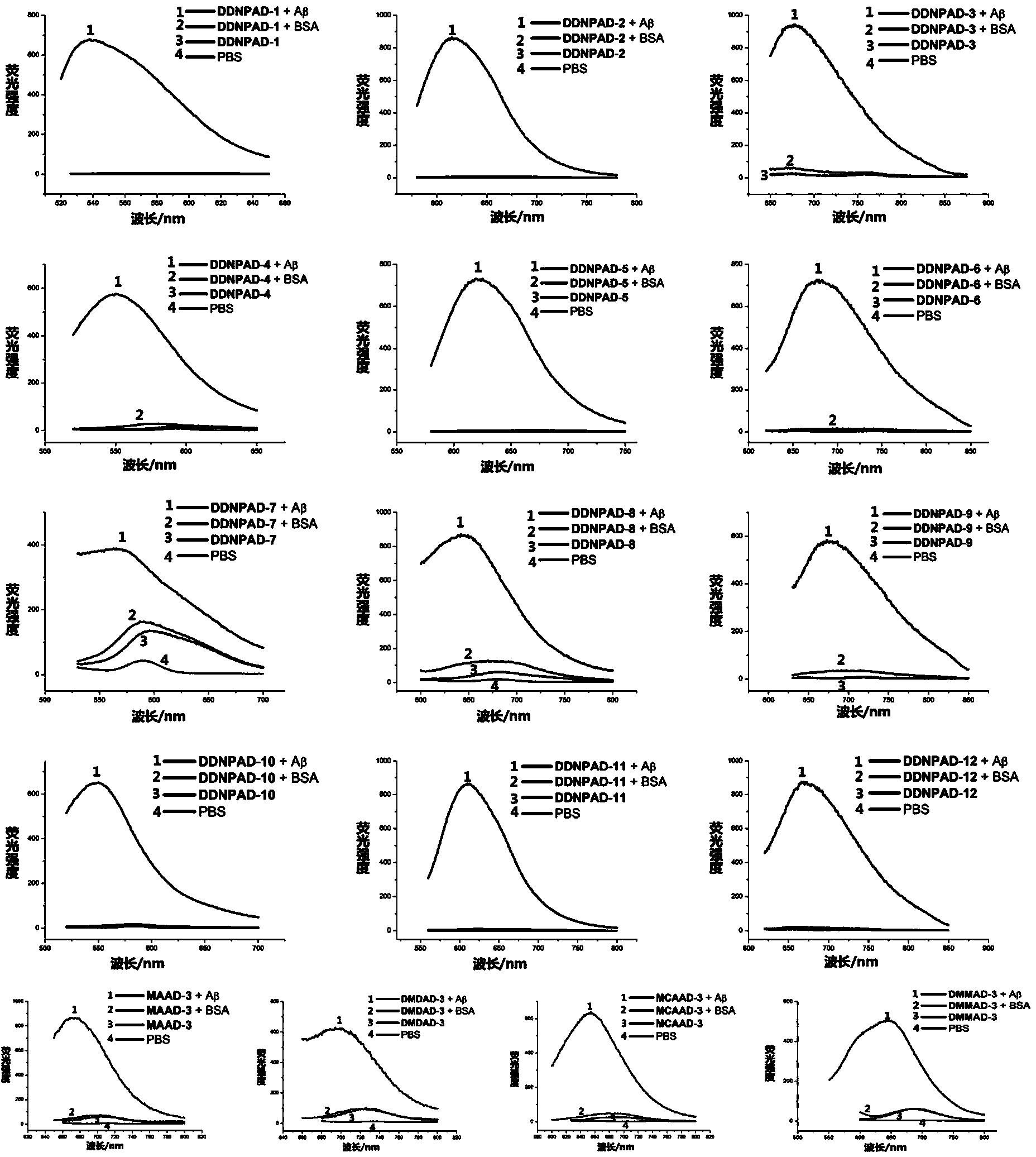
[0188] Example 3 Effect Experiment of Fluorescent Probe Molecules
[0189] 1. Determination of excitation and emission spectra of fluorescent probe molecules
[0190] 1.1 Experimental steps
[0191] (1) prepare the dichloromethane solution of compound, concentration is 10 -3 mol / L (1 mmol / L).
[0192] (2) Preparation of compound organic solution. Take 30 μL of the above solution and dilute to 3 mL with different organic solvents such as dichloromethane, tetrahydrofuran, methanol and DMSO to obtain a 10 μM solution.
[0193] (3) Preparation of compound PBS solution. Take 6 μL of the mother solution in step (1) with a microsampler, volatilize the dichloromethane, add 50 μL DMSO to dissolve the compound, then add 250 μL ethanol, add PBS buffer solution to 3 mL, and finally obtain a 2 μM PBS solution of the compound (containing 10 % ethanol).
[0194] (4) Fluorescence excitation and emission spectra of compounds were measured and recorded with RF-5301PC fluorescence spectrom...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com